Inhibition of host pre-mRNA processing by virus (kw:KW-1103)
Host mRNAs poly(A) tail is added in two steps. First, pre-mRNAs are cleaved and a short poly(A) tail is added. Subsequent elongation of this short poly(A) tail requires poly(A)-binding protein II, CPSF complex and PAP (PABII), which facilitate rapid, processive poly(A) addition. PABII also plays a role in nuclear export of host mRNAs.
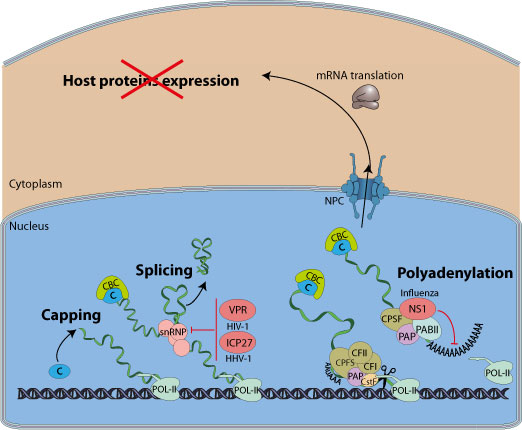
Some viruses interfere with host pre-mRNA processing function. Incompletely processed mRNA are non-functional leading to shutoff of host proteins expression. This gives viruses transcripts a competitive edge for access to the cellular translation machinery. Preventing the expression of host proteins is also a strategy to counteract the antiviral response.
Some viruses have been shown to inhibit host pre-mRNA processing by blocking cellular splicing or polyadenylation.
Herpesvirus protein ICP27 mediates the inhibition of cellular splicing. Early in infection, ICP27 interacts with several splicing factors, including members of a family of essential splicing factors termed SR proteins, and affects their phosphorylation. This results in the blockage of the pre-spliceosome assembly, which in turn contributes to the shutoff of host protein synthesis because of incomplete cellular pre-mRNAs processing.
Influenza virus NS1 can bind to CPSF30 and PABII and thus inhibits host pre-mRNA processing.
NS1 of the 2009 Pandemic H1N1 has a truncated C-terminus and has apparently lost the ability to bind PABII 
Viruses inhibiting host mRNA splicing:
| Family | Virus | Viral protein | mRNA processing inhibition strategy | references |
| Orthomyxoviridae | Influenza A virus | NS1 | Inhibition of polyadenylation via binding to CPSF30 and PABII |



|
| Herpesviridae | HHV1, HHV2 | ICP27 | Inhibition of splicing |



|
| Retroviridae | HIV-1 | VPR | Inhibition of splicing |

|
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)0 entry grouped by protein
