Apoptosis (or programmed cell death) is a controlled process leading to cell death characterized by chromatin condensation, DNA fragmentation, cell shrinkage, and compartmentalization of the dead cells to apoptotic bodies. The host immune system uses apoptosis to eliminate cells infected by pathogens. Apoptosis of infected cells is caused either by cytolytic cells activated during the anti-viral response, or directly by viral infection. Host caspases, constitute an evolutionary conserved family of aspartate-specific cysteine proteases that are at the heart of the apoptotic machinery.
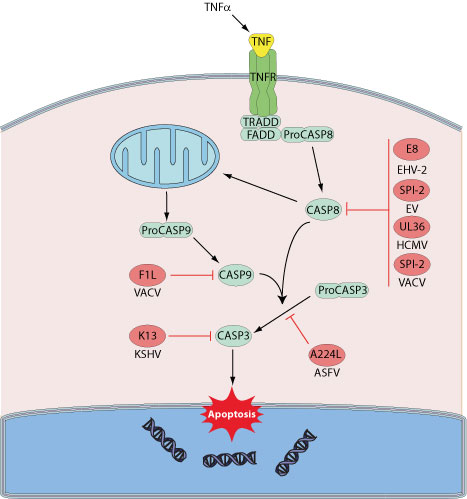
Playing a central role in cell death, caspases are targeted by different viruses in the aim of prolonging cell survival. For instance, viral IAPs (inhibitors of apoptosis) interact with and inhibit the processed active caspases, either by blocking the catalytic part of the enzymes, or through E3 ubiquitin ligase activity from RING domains, targeting caspases for rapid degradation via proteasome. Baculoviruses, asfiviruses or irridescent viruses encode such IAPs.
Another class of caspase inhibitors frequently found among poxviruses include the serine proteinase inhibitors serpins: CrmA/SPI-2. Crma targets cysteine proteases such as host Caspase 1 and Caspase 8.

