COVID-19 vaccines
Source: 
mRNA vaccines
|
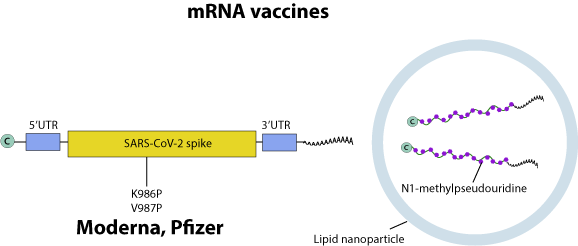
mRNA vaccines are composed of a antigen-encoding messenger RNA encapsulated into a lipid micelle.
Production: The antigen gene is encoded in a DNA plasmid that is transcribed in vitro by T7 RNA polymerase to generate mRNA. During transcription, uridines are replaced with N1-methylpseudouridine to stabilize the molecule. The mRNA is capped (cellular or vaccinia virus capping enzyme) and polyadenylated (polyA polymerase). mRNAs are then purified, a step that may involve DNAse digestion to remove the original plasmid DNA. The purified mRNAs are then encapsulated in a lipid envelope.
The protein contains two proline mutations in S2 (K986P and V987P) to stabilize the trimer.
Vaccination: Intra-muscular. After injection, the encapsulated mRNAs either enter any cells or are scavenged by immune cells such as dendritic cells. In both cases, the mRNA that enters the cytoplasm is translated and the antigen is expressed, triggering an immune response.
Dosage 30?microgram RNA (2x) (BioNTech-Pfizer), 100 microgram RNA (2x) (Moderna-NIAID)
|
|
Adenovirus vector vaccine
|
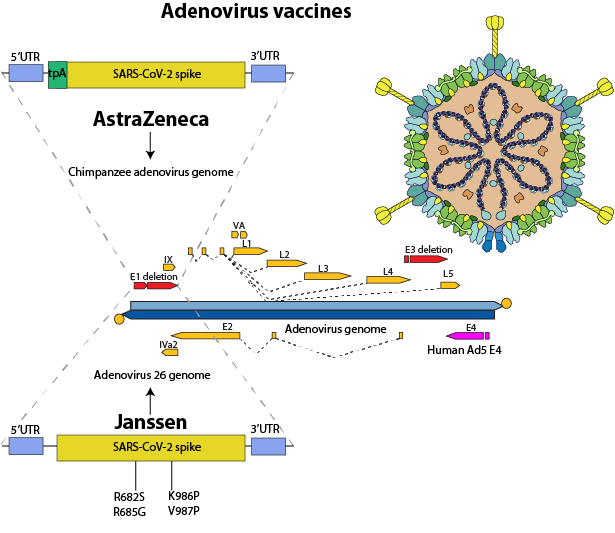
Adenovirus vector vaccines consist of adenovirus particles (dsDNA, non-enveloped, replicated in the nucleus) encoding an antigen.
Production: The spike gene was inserted into the E1 region of a chimpanzee adenovirus ChAd Y25 genome (AstraZeneca) or an adenovirus type 26 genome (Janssen). The adenovirus vector genome was modified as follows:
-Deletion of the E1 region renders the vector replication incompetent in non-complementary cells such as human cells,
-Deletion of the E3 region creates sufficient space in the viral genome for the insertion of foreign antigens,the replacement of -E4 ORF6 exchanged by the Ad5 homologue allows the production of replication-incompetent vectors in Ad5 E1 complementing cell lines.
-The viruses are grown in culture tanks on HEK293 (AstraZeneca) or PER.C6 cells (Janssen).
Janssen: The protein contains two proline mutations in S2 (K986P and V987P) to stabilize the trimer, plus mutations in the S1-S2 cleavage site (R682S and R685G).
Vaccination: Intra-muscular. After injection, the adenoviral virions enter the target cells, their genome is translocated to the nucleus where the antigen mRNA is produced by cellular machinery (see wild type adenovirus replication cycle). The mRNA is translated and the antigen is expressed, triggering an immune response.
Dosage: 5.1010 adenovirus vector particles (2x) (University of Oxford-AstraZeneca) and (Janssen-Johnson & Johnson)
|
|
Inactivated vaccine
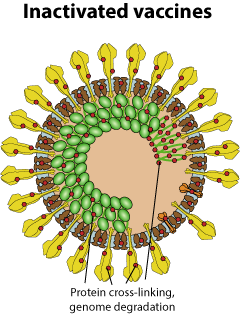
Inactivated vaccines consist of true virions rendered useless by chemical or UV treatment.
Production: The virus is grown in Vero cells, purified and inactivated with beta-propiolactone (BPL) (Sinopharm and Sinovac). This causes protein cross-linking and irreversible alkylation of the nucleic acid bases, completely suppressing transcription and replication of the viral genome  . The resulting virions are too damaged to cause infection but retain their antigenic properties.
Vaccination Intra-muscular. After injection, the inactivated virions are directly exposed to the immune system.
Dosage 3 microgram proposed (2x) (Sinovac), . The resulting virions are too damaged to cause infection but retain their antigenic properties.
Vaccination Intra-muscular. After injection, the inactivated virions are directly exposed to the immune system.
Dosage 3 microgram proposed (2x) (Sinovac),
|
|
subunit vaccine
|
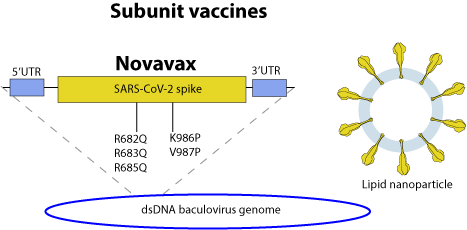
Subunit vaccines consist only of purified antigen protein.
Production: The full length trimeric spike is produced as a recombinant protein in sf9 cells using a baculovirus (insect virus) expression system. The trimers of spike are purified and mixed with lipid to form nanoparticles.
Novavax: The protein contains two proline mutations in S2 (K986P and V987P) to stabilize the trimer, plus mutations in the S1-S2 cleavage site (R682Q, R683Q and R685Q).
Vaccination: Intra-muscular. After injection, the antigens are directly exposed to the immune system.
Dosage 5 microgram S (+50 microgram adjuvant) (2x) (Novavax)
|
|
Inactivation methods for whole influenza vaccine production
Ailar Sabbaghi, Seyed Mohammad Miri, Mohsen Keshavarz, Mohsen Zargar, Amir Ghaemi
Rev Med Virol. 2019 Nov;29(6)
Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action
Franz X. Heinz & Karin Stiasny
npj Vaccines volume 6, Article number: 104 (2021)


 . The resulting virions are too damaged to cause infection but retain their antigenic properties.
Vaccination Intra-muscular. After injection, the inactivated virions are directly exposed to the immune system.
Dosage 3 microgram proposed (2x) (Sinovac),
. The resulting virions are too damaged to cause infection but retain their antigenic properties.
Vaccination Intra-muscular. After injection, the inactivated virions are directly exposed to the immune system.
Dosage 3 microgram proposed (2x) (Sinovac),


