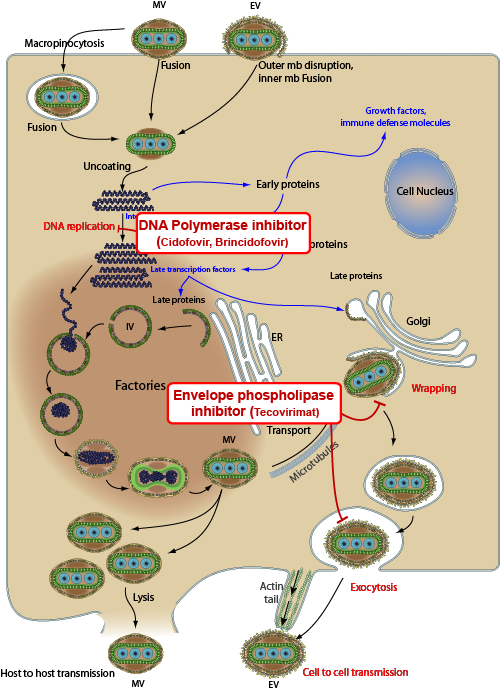
Antiviral drugs
Sources: CDC Smallpox Prevention and Treatment
An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge
Guang Yang, Daniel C Pevear, Marc H Davies, Marc S Collett, Tom Bailey, Susan Rippen, Linda Barone, Chris Burns, Gerry Rhodes, Sanjeev Tohan, John W Huggins, Robert O Baker, R L Mark Buller, Erin Touchette, Kem Waller, Jill Schriewer, Johan Neyts, Erik DeClercq, Kevin Jones, Dennis Hruby, Robert Jordan
J Virol. 2005 Oct;79(20):13139-49.