Inhibition of host mRNA nuclear export by virus (kw:KW-1099)
The nuclear export of cellular mRNAs is mediated by factors interacting with both the messenger ribonucleoprotein (mRNP) and the nucleoporins (Nup) to deliver the mRNAs through the nuclear pore complex to the cytoplasm.
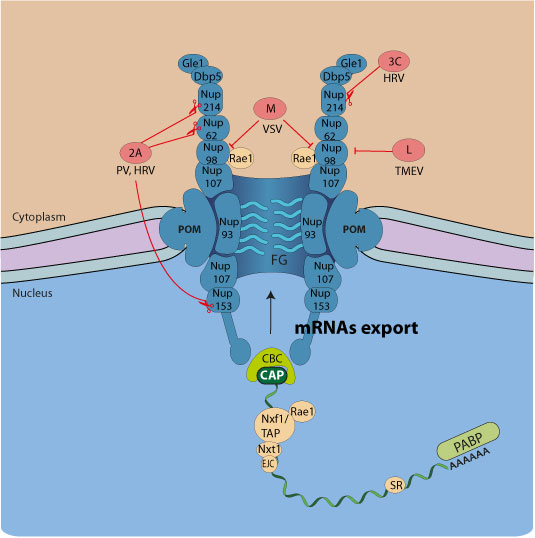
Several viruses interfere with host mRNA nucleo-cytoplasmic trafficking, eventually leading to shutoff of host proteins expression. This gives viruses transcripts a competitive edge for access to the cellular translation machinery. Preventing the expression of host proteins is also a strategy to counteract the antiviral response.
For example, VSV matrix protein inhibits mRNA export by forming a complex with the mRNA export factor Rae1, whereas the 2A protease of picornaviruses promotes cleavage of host Nup98, Nup153, Nup62  .
.
Viruses inhibiting host mRNA nuclear export:
| Family | Virus | Viral protein | mRNA nuclear export inhibition strategy | references |
| Picornaviridae | Poliovirus (Enterovirus) | 2A protease | Breakdown of Nup62, Nup98, Nup153 |


|
| HRV2, HRV14, HRV16 (Enterovirus) | 2A protease | Breakdown of Nup62, Nup98, Nup153 |


 |
|
| HRV (Enterovirus) | 3A protease | Breakdown of Nup153, Nup214, and Nup358 |

| |
| Theiler's virus (Cardiovirus) | Leader protein | Hyperphosphorylation of Nup98 |

|
|
| Encephalomyocarditis virus (Cardiovirus) | Leader protein | Hyperphosphorylation of Nup62, Nup153, and Nup214 |


|
|
| Rhabdoviridae | Vesicular stomatitis virus (Vesiculovirus) | M protein | Disrupts Rae1-Nup98 interaction |


|
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)59 entries grouped by strain
1 entry
Aichi virus (strain Human/A846/88/1989) (AiV) (Aichi virus (strain A846/88)) reference strain
1 entry
Encephalomyocarditis virus (strain Rueckert) (EMCV) reference strain
1 entry
Human rhinovirus A serotype 89 (strain 41467-Gallo) (HRV-89) reference strain
1 entry
Poliovirus type 1 (strain Mahoney) reference strain
1 entry
Salivirus A (isolate Human/Nigeria/NG-J1/2007) (SV-A) reference strain
1 entry
Vesicular stomatitis Indiana virus (strain San Juan) (VSIV) reference strain
1 entry
Vesicular stomatitis New Jersey virus (strain Ogden subtype Concan) (VSNJV) reference strain
1 entry
Bovine enterovirus (strain VG-5-27) (BEV)
1 entry
Coxsackievirus A16 (strain G-10)
1 entry
Coxsackievirus A16 (strain Tainan/5079/98)
1 entry
Coxsackievirus A21 (strain Coe)
1 entry
Coxsackievirus A24 (strain EH24/70)
1 entry
Coxsackievirus A9 (strain Griggs)
1 entry
Coxsackievirus B1 (strain Japan)
1 entry
Coxsackievirus B2 (strain Ohio-1)
1 entry
Coxsackievirus B3 (strain Nancy)
1 entry
Coxsackievirus B3 (strain Woodruff)
1 entry
Coxsackievirus B4 (strain E2)
1 entry
Coxsackievirus B4 (strain JVB / Benschoten / New York/51)
1 entry
Coxsackievirus B5 (strain Peterborough / 1954/UK/85)
1 entry
Coxsackievirus B6 (strain Schmitt)
1 entry
Echovirus 1 (strain Human/Egypt/Farouk/1951) (E-1)
1 entry
Echovirus 11 (strain Gregory)
1 entry
Echovirus 12 (strain Travis)
1 entry
Echovirus 30 (strain Bastianni)
1 entry
Echovirus 5 (strain Noyce)
1 entry
Echovirus 6 (strain Charles)
1 entry
Echovirus 9 (strain Barty)
1 entry
Echovirus 9 (strain Hill)
1 entry
Encephalomyocarditis virus
1 entry
Encephalomyocarditis virus (strain emc-b nondiabetogenic)
1 entry
Encephalomyocarditis virus (strain emc-d diabetogenic)
1 entry
Human enterovirus 70 (strain J670/71) (EV70) (EV-70)
1 entry
Human enterovirus 71 (EV71) (EV-71)
1 entry
Human enterovirus 71 (strain 7423/MS/87) (EV71) (EV-71)
1 entry
Human enterovirus 71 (strain USA/BrCr/1970) (EV71) (EV-71)
1 entry
Human enterovirus D68 (EV68) (EV-68)
1 entry
Human klassevirus 1 (HKV-1)
1 entry
Human rhinovirus 14 (HRV-14)
1 entry
Human rhinovirus 16 (HRV-16)
1 entry
Human rhinovirus 1A (HRV-1A)
1 entry
Human rhinovirus 1B (HRV-1B)
1 entry
Human rhinovirus 2 (HRV-2)
1 entry
Human rhinovirus 3 (HRV-3)
1 entry
Human rhinovirus C (strain C15) (HRV-C15)
1 entry
Poliovirus type 1 (strain Sabin)
1 entry
Poliovirus type 2 (strain Lansing)
1 entry
Poliovirus type 2 (strain W-2)
1 entry
Poliovirus type 3 (strain 23127)
1 entry
Poliovirus type 3 (strains P3/Leon/37 and P3/Leon 12A[1]B)
1 entry
Porcine enterovirus 9 (strain UKG/410/73)
1 entry
Swine vesicular disease virus (strain H/3 '76) (SVDV)
1 entry
Swine vesicular disease virus (strain UKG/27/72) (SVDV)
1 entry
Theiler's murine encephalomyelitis virus (strain GDVII) (TMEV)
1 entry
Venezuelan equine encephalitis virus (strain Trinidad donkey) (VEEV)
1 entry
Vesicular stomatitis Indiana virus (strain 85CLB South America) (VSIV)
1 entry
Vesicular stomatitis Indiana virus (strain 94GUB Central America) (VSIV)
1 entry
Vesicular stomatitis Indiana virus (strain 98COE North America) (VSIV)
1 entry
