Enterovirus (taxid:12059)
NB. Rhinoviruses are merged into enterovirus genus
VIRION
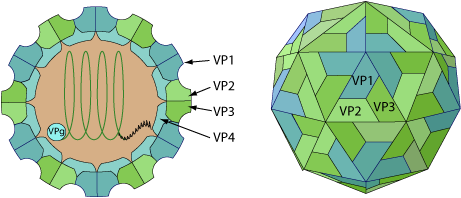
Non-enveloped, spherical, about 30 nm in diameter, T=pseudo3 icosahedral capsid surrounding the naked RNA genome. The capsid consists of a densely-packed icosahedral arrangement of 60 protomers, each consisting of 4 polypeptides, VP1, VP2, VP3 and VP4. VP4 is located on the internal side of the capsid.
GENOME
Monopartite, linear ssRNA(+) genome of 7.2-8.5 kb, polyadenylated, composed of a single ORF encoding a polyprotein. Viral genomic RNA has a viral protein (VPg) at its 5' end instead of a methylated nucleotide cap structure. The long UTR at the 5' end contains a type I internal ribosome entry site (IRES). The P1 region encodes the structural polypeptides. The P2 and P3 regions encode the nonstructural proteins associated with replication.
GENE EXPRESSION
The virion RNA is infectious and serves as both the genome and viral messenger RNA. The IRES allows direct translation of the polyprotein. The polyprotein is initially processed by the viral proteases into three precursor proteins, P1, P2, and P3. Precursor P1 is then proteolytically cleaved to yield the structural proteins. Precursors P2 and P3 are processed into replicase, VPg, and a number of proteins that modify the host cell, ultimately leading to cell lysis. Enterovirus A, B, E, F and G genomes and around half the enterovirus C genomes encode an upstream open reading frame (uORF).
ENZYMES
- RNA-dependent RNA polymerase [RdRp]
- VPG-type capping [VPg]
- NTPase-helicase [2C]
- Polyprotein major protease (Peptidase C3) [3Cpro, 2A]
- Virion maturation (Peptidase N8) [VP4]
REPLICATION
CYTOPLASMIC
- Attachement of the virus to host receptors mediates endocytosis of the virus into the host cell.
- The capsid undergoes a conformational change and releases VP4 that opens a pore in the host endosomal membrane and the viral genomic RNA penetrates into the host cell cytoplasm (the empty capsid remains intact).
- VPg is removed from the viral RNA, which is then translated into a processed polyprotein.
- Shutoff of cellular cap-dependent translation through the cleavage of translation initiation factors by viral protease.
- Replication occurs in viral factories made of membrane vesicles derived from the ER. A dsRNA genome is synthesized from the genomic ssRNA(+).
- The dsRNA genome is transcribed/replicated thereby providing viral mRNAs/new ssRNA(+) genomes.
- New genomic RNA is believed to be packaged into preassembled procapsids.
- Cell lysis and virus release.
- Maturation of provirions by an unknown host protease.
Host-virus interaction
Apoptosis modulation
Picornaviruses modulate host apoptosis  .
.
Poliovirus infection activates the apoptotic pathway, involving mitochondrial damage, cytochrome c efflux, and consecutive activation of caspases (caspase-3 caspase and -9)  , whereas antiapoptotic activity has been attributed to cardiovirus leader protein
, whereas antiapoptotic activity has been attributed to cardiovirus leader protein  .
.
Modulation of apoptosis depends on the host cell-type and on the time the infection because of the presence of viral pro- and anti-apoptotic factors respectively at the beginning and at the end of the infectious cycle


 .
.
Autophagy modulation
Picornaviruses subvert the cell autophagic pathway to facilitates viral infection  :
:
- Human rhinovirus 2 activates the autophagic pathway
- Coxsackievirus and poliovirus infection induces autophagy-like vesicles


Innate immune response inhibition
Picornaviruses inhibit the host IFN-mediated response by inactivating either MDA-5, RIG-I, MAVS or IRF-3:
- Coxsackievirus B3 cleaves and inhibits MAVS 
- Enteroviruses cleave and inhibit RIG-I

- Poliovirus cleaves and inhibits MDA-5

Picornaviruses inhibit Toll-like receptor 3 mediated antiviral response as well:
- Coxsackievirus B 3C and enterovirus 71 proteases 3C cleave TRIF

 .
.
Host gene expression shutoff by virus
Enteroviruses inhibit host translation by cleaving
PABP (coxsackievirus and poliovirus) 
 , eIF5B
, eIF5B  and activating 4E-BP1
and activating 4E-BP1  .
.
Poliovirus also inhibits host transcription by cleaving the TATA-binding protein  .
.
HRV14, HRV16 and poliovirus inhibit host mRNA export as well


 .
.
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)47 entries grouped by protein
3 entries
ORF2p protein
44 entries
