Inhibition of host NF-kappa-B by virus (kw:KW-1100)
NF-kappa-B is a pleiotropic transcription factor which is present in almost all cell types and is involved in many biological processed such as inflammation, immunity, differentiation, cell growth, tumorigenesis and apoptosis. In unstimulated cells, NF-kappa-B dimers are sequestered in the cytoplasm via physical association with NF-kappa-B inhibitory proteins, called I-kappa-Bs. Upon activation, NF-kappa-B separates from I-kappa-B and migrates to the nucleus to activate gene transcription.
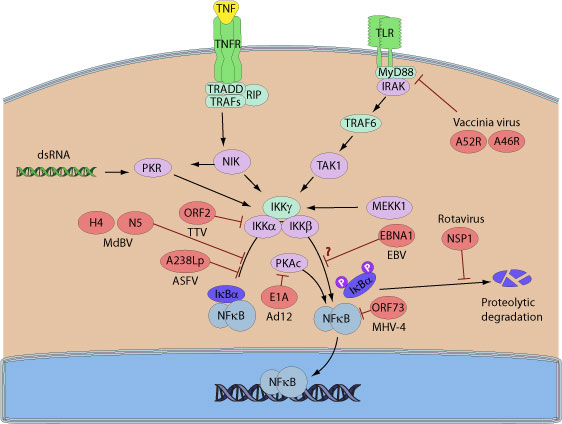
Given the importance of controlling NF-kappa-B during viral infection, many viruses encode proteins that act on different cellular targets to ultimately inhibit the NF-kappa-B pathway. For example, several large DNA viruses including african swine virus, vaccinia virus or bracovirus encode proteins containing ankyrin repeats able to inhibit NF-kappa-B. Indeed, Vaccinia K1 inhibits NF-kappa-B by preventing I-kappa-B degradation. A238L from African swine fever virus interacts with free NF-kappa-B to prevent its nuclear translocation. Microplitis demolitor bracovirus encodes I-kappa-B-like genes. These viral proteins function as I-kappa-Bs and acts as inhibitors of NF-kappa-B, the HIV accessory protein Vpu was shown to inhibit NF-kappa-B activation by interfering with b-TrCP-mediated degradation of I-kappa-B and to promote apoptosis of the infected cell by suppressing NF-kBdependent expression of anti-apoptotic factors.
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)149 entries grouped by strain
12 entries
Microplitis demolitor bracovirus (isolate Webb) (MdBV) reference strain
8 entries
Vaccinia virus (strain Western Reserve) (VACV) (Vaccinia virus (strain WR)) reference strain
7 entries
Monkeypox virus (MPXV) reference strain
5 entries
African swine fever virus (strain Badajoz 1971 Vero-adapted) (Ba71V) (ASFV) reference strain
4 entries
Variola virus (isolate Human/India/Ind3/1967) (VARV) (Smallpox virus) reference strain
3 entries
Molluscum contagiosum virus subtype 1 (MOCV) (MCVI) reference strain
2 entries
Bovine respiratory syncytial virus (strain A51908) (BRS) reference strain
2 entries
Human respiratory syncytial virus B (strain B1) reference strain
1 entry
Bat coronavirus HKU4 (BtCoV) (BtCoV/HKU4/2004) reference strain
1 entry
Bat coronavirus HKU5 (BtCoV) (BtCoV/HKU5/2004) reference strain
1 entry
Bat coronavirus HKU9 (BtCoV) (BtCoV/HKU9) reference strain
1 entry
Borna disease virus (strain V) (BDV) reference strain
1 entry
Cowpox virus (strain Brighton Red) (CPV) reference strain
1 entry
Epstein-Barr virus (strain AG876) (HHV-4) (Human herpesvirus 4) reference strain
1 entry
Epstein-Barr virus (strain B95-8) (HHV-4) (Human herpesvirus 4) reference strain
1 entry
Human adenovirus A serotype 12 (HAdV-12) (Human adenovirus 12) reference strain
1 entry
Human adenovirus C serotype 2 (HAdV-2) (Human adenovirus 2) reference strain
1 entry
Human coronavirus HKU1 (isolate N1) (HCoV-HKU1) reference strain
1 entry
Human coronavirus OC43 (HCoV-OC43) reference strain
1 entry
Human cytomegalovirus (strain AD169) (HHV-5) (Human herpesvirus 5) reference strain
1 entry
Human cytomegalovirus (strain Merlin) (HHV-5) (Human herpesvirus 5) reference strain
1 entry
Human enterovirus D68 (EV68) (EV-68) reference strain
1 entry
Human herpesvirus 8 type P (isolate GK18) (HHV-8) (Kaposi's sarcoma-associated herpesvirus) reference strain
1 entry
Mumps virus genotype B (strain Miyahara vaccine) (MuV) reference strain
1 entry
Murid herpesvirus 1 (strain Smith) (MuHV-1) (Mouse cytomegalovirus) reference strain
1 entry
Murine coronavirus (strain A59) (MHV-A59) (Murine hepatitis virus) reference strain
1 entry
Orf virus (strain Goat/Texas/SA00/2000) (OV-SA00) (Orf virus-San Angelo 2000) reference strain
1 entry
Porcine epidemic diarrhea virus (strain CV777) (PEDV) reference strain
1 entry
Porcine reproductive and respiratory syndrome virus (strain Lelystad) (PRRSV) reference strain
1 entry
Porcine reproductive and respiratory syndrome virus (strain VR-2332) (PRRSV) reference strain
1 entry
Severe acute respiratory syndrome coronavirus (SARS-CoV) reference strain
1 entry
Severe acute respiratory syndrome coronavirus 2 (2019-nCoV) (SARS-CoV-2) reference strain
6 entries
Vaccinia virus (strain Copenhagen) (VACV)
4 entries
African swine fever virus (isolate Pig/Kenya/KEN-50/1950) (ASFV)
4 entries
African swine fever virus (isolate Tick/South Africa/Pretoriuskop Pr4/1996) (ASFV)
3 entries
African swine fever virus (isolate Tick/Malawi/Lil 20-1/1983) (ASFV)
3 entries
African swine fever virus (isolate Warthog/Namibia/Wart80/1980) (ASFV)
3 entries
Middle East respiratory syndrome-related coronavirus (isolate United Kingdom/H123990006/2012) (MERS-CoV) (Betacoronavirus England 1)
2 entries
Human respiratory syncytial virus
2 entries
Human respiratory syncytial virus A (strain A2)
2 entries
Human respiratory syncytial virus B (strain 18537)
2 entries
Variola virus
1 entry
Bat coronavirus 133/2005 (BtCoV) (BtCoV/133/2005)
1 entry
Bat coronavirus 279/2005 (BtCoV) (BtCoV/279/2005)
1 entry
Bat coronavirus HKU3 (BtCoV) (SARS-like coronavirus HKU3)
1 entry
Bat coronavirus Rp3/2004 (BtCoV/Rp3/2004) (SARS-like coronavirus Rp3)
1 entry
Bovine coronavirus (strain 98TXSF-110-ENT) (BCoV-ENT) (BCV)
1 entry
Bovine coronavirus (strain 98TXSF-110-LUN) (BCoV-LUN) (BCV)
1 entry
Bovine coronavirus (strain Mebus) (BCoV) (BCV)
1 entry
Bovine coronavirus (strain Quebec) (BCoV) (BCV)
1 entry
Bovine respiratory syncytial virus (strain 391-2) (BRS)
1 entry
Bovine respiratory syncytial virus (strain Rb94) (BRS)
1 entry
Cowpox virus (CPV)
1 entry
Coxsackievirus A16 (strain G-10)
1 entry
Coxsackievirus A16 (strain Tainan/5079/98)
1 entry
Epstein-Barr virus (strain GD1) (HHV-4) (Human gammaherpesvirus 4)
1 entry
Human coronavirus HKU1 (isolate N2) (HCoV-HKU1)
1 entry
Human coronavirus HKU1 (isolate N5) (HCoV-HKU1)
1 entry
Mumps virus (strain Belfast) (MuV)
1 entry
Mumps virus (strain Bristol 1) (MuV)
1 entry
Mumps virus (strain Edingburgh 2) (MuV)
1 entry
Mumps virus (strain Edingburgh 4) (MuV)
1 entry
Mumps virus (strain Edingburgh 6) (MuV)
1 entry
Mumps virus (strain Enders) (MuV)
1 entry
Mumps virus (strain Kilham) (MuV)
1 entry
Mumps virus (strain Matsuyama) (MuV)
1 entry
Mumps virus (strain RW) (MuV)
1 entry
Mumps virus (strain SBL) (MuV)
1 entry
Mumps virus (strain SBL-1) (MuV)
1 entry
Mumps virus (strain Takahashi) (MuV)
1 entry
Mumps virus genotype A (strain Jeryl-Lynn) (MuV)
1 entry
Mumps virus genotype B (strain Urabe vaccine AM9) (MuV)
1 entry
Murine coronavirus (strain 2) (MHV-2) (Murine hepatitis virus)
1 entry
Murine coronavirus (strain JHM) (MHV-JHM) (Murine hepatitis virus)
1 entry
Porcine reproductive and respiratory syndrome virus (PRRSV)
1 entry
Porcine reproductive and respiratory syndrome virus (isolate Pig/United States/SD 01-08/2001) (PRRSV)
1 entry
Porcine reproductive and respiratory syndrome virus (strain 16244B) (PRRSV)
1 entry
Porcine reproductive and respiratory syndrome virus (strain HB-1) (PRRSV)
1 entry
Rotavirus A (isolate RVA/Human/India/116E/1986/G9P8[11]) (RV-A)
1 entry
Rotavirus A (isolate RVA/Human/Japan/IGV-80-3/XXXX/GXP[X]) (RV-A)
1 entry
Rotavirus A (isolate RVA/Human/United States/WI61/1983/G9P1A[8]) (RV-A)
1 entry
Rotavirus A (strain RVA/Human/Indonesia/69M/1980/G8P4[10]) (RV-A)
1 entry
Rotavirus A (strain RVA/Human/Japan/K8/1977/G1P3A[9]) (RV-A)
1 entry
Rotavirus A (strain RVA/Human/Japan/KU/1995/G1P1A[8]) (RV-A)
1 entry
Rotavirus A (strain RVA/Human/Philippines/L26/1987/G12P1B[4]) (RV-A)
1 entry
Rotavirus A (strain RVA/Human/United Kingdom/ST3/1975/G4P2A[6]) (RV-A) (Rotavirus A (strain St. Thomas 3))
1 entry
Rotavirus A (strain RVA/Human/United States/D/1974/G1P1A[8]) (RV-A)
1 entry
Rotavirus A (strain RVA/Human/United States/P/1974/G3P1A[8]) (RV-A)
1 entry
Rotavirus A (strain RVA/Human/United States/Wa/1974/G1P1A[8]) (RV-A)
1 entry
Rotavirus A (strain RVA/Human/Venezuela/M37/1982/G1P2A[6]) (RV-A)
1 entry
Rotavirus A (strain RVA/Pig/Mexico/YM/1983/G11P9[7]) (RV-A)
1 entry
Rotavirus A (strain RVA/Pig/United States/Gottfried/1983/G4P2B[6]) (RV-A)
1 entry
