Inhibition of host apoptosis by viral FLIP-like protein (kw:KW-1082)
Apoptosis (or programmed cell death) is a controlled process leading to cell death characterized by chromatin condensation, DNA fragmentation, cell shrinkage, and compartimentalization of the dead cells to apoptotic bodies. The host immune system uses apoptosis to eliminate cells infected by pathogens. Apoptosis of infected cells is caused either by cytolytic cells activated during the anti-viral response, or directly by viral infection.
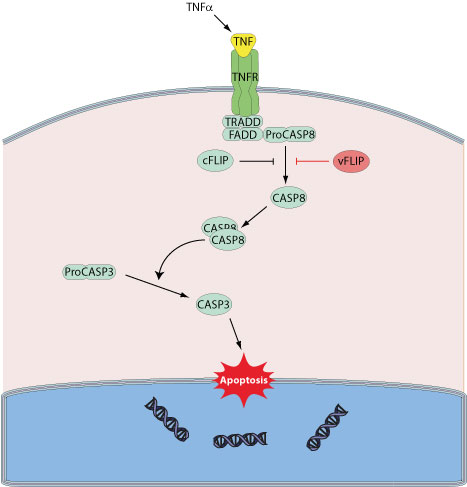
Many viruses have evolved mechanisms to either inhibit or activate cell death depending on their needs. Inhibition of apoptosis allow viruses to optimize replication and progeny synthesis by prolonging the infected cell life. Alternatively, viruses may trigger host cell apoptsis to release progeny virions. Several large DNA viruses encode viral FLIP proteins, sharing strong sequence similarites with host c-FLIP, a protein that acts as an inhibitor of TNFRSF6 mediated apoptosis. Similar to c-FLIPs, the vFLIPs can block the interaction of the death receptor-adapter complex with the cellular effector FLICE (caspase-8), and this prevents the initiation of the downstream caspase cascade.
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)3 entries grouped by protein
2 entries
Viral CASP8 and FADD-like apoptosis regulator (v-CFLAR) (Viral FLICE-inhibitory protein) (v-FLIP)
1 entry
