HBx
HBx is restrained to mammalian virus
The HBx open reading frame exists only in HBV infecting mammals. A putative X protein was described for DHBV (Chang et al. 2001) Rating=0 , but has no apparent function in vitro or in vivo (Meier et al. 2003) Rating=2. The X protein would therefore be necessary only in mammals.
comments: Mammalian and particularly primate retroviruses encode for supplemental proteins compared to avian retroviruses. Most of them counteract host defenses which are stronger in mammals, presumably due to the evolution history which implies many endogenous retroviruses. HBV would be similar in the fact that the X would be necessary to circumvent mammals specific cellular defenses.
HBx Characterization
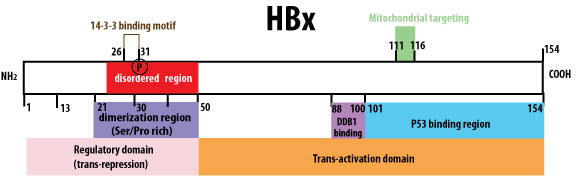
Murakami et al. 1994 (trans-repression region), Li et al. 2009 (DDB1-binding motif), Li et al. 2008 (mitochondrial targeting region), Diao et al. 2008, Elmore et al. 1997 (P53 binding region), Tang et al. 2005
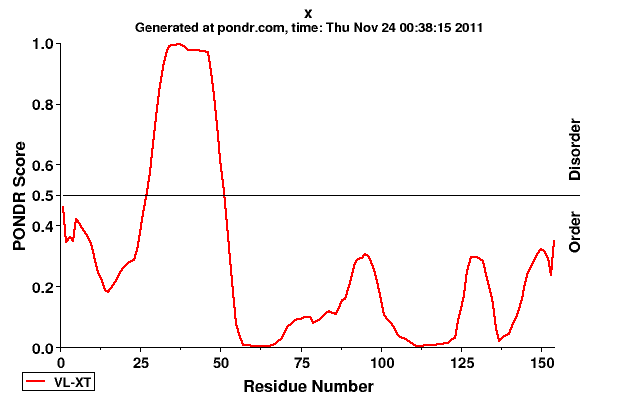
Localization
HBx is primarily localized in the nucleus at low expression levels but accumulates in the cytoplasm at elevated HBx levels (Cha et al. 2009). HBx shuttles between the cytoplasm and the nucleus through a Crm-1-dependent nuclear export pathway (Forgues et al. 2001). HBx has been detected in the mitochondria as well (Li et al. 2008).
HBx turnover
HBx is expressed early in the replication cycle (Wu et al. 1991) Rating=2, (Doitsh et al. 2004) Rating=2.
HBx can be ubiquitinated and degraded through the ubiquitin-proteasome pathway. It has been proposed that degradation of HBx is stimulated by HBV core protein (HBc) (Kim et al. 2003) Rating=1, by Hdj1 (Sohn et al. 2006) Rating=1. More recently, Siah, a novel E3 ubiquitin ligase for HBx, has been shown to interact with HBx and facilitates HBx poly-ubiquitylation and proteasomal degradation (Zaho et al. 2011) Rating=1. TSPX tumor suppressor has also been implicated in HBx degradation (Kido et al. 2011) Rating=1.
HBx and Hepatocellular Carcinomas
HBx transactivates viral (Zaho et al. 2011) and cellular genes expression either by interacting with various transcription factors, coactivators, and components of the basal transcription machinery in the nucleus or by activating signaling pathways in the cytoplasm (Ma et al. 2011) Rating=Review. These pathways seem to globally upregulate viral replication and cell survival/proliferation. As a consequence, HBx probably participates in inflammation/fibrosis processes and hepatocellular carcinoma (HCC) development (Brechot et al. 2010) Rating=Review. . The viral protein mostly integrated in the chromosomal DNA of patients with HCC is HBx (Paterlini et al. 1995) Rating=1.