Nucleoside Reverse Transcriptase Inhibitors
Approved, nucleoside analogs:
Abacavir (ABC)![]()
 ,
Didanosine (ddI)
,
Didanosine (ddI)![]()
 ,
Emtricitabine (FTC)
,
Emtricitabine (FTC)![]()
 ,
Lamivudine (3TC)
,
Lamivudine (3TC)![]()
 ,
Stavudine (d4T)
,
Stavudine (d4T)![]()
 ,
Zidovudine/Azidothymidine (AZT)
,
Zidovudine/Azidothymidine (AZT)![]()
 ,
Zalcitabine (ddC)
,
Zalcitabine (ddC)![]() Approved, Cytosine analogues:
Tenofovir Disoproxil Fumarate (TDF)
Approved, Cytosine analogues:
Tenofovir Disoproxil Fumarate (TDF)![]() !(image)./resources/AIDSinfo.gif!,
Tenofovir alafenamide (TAF)
!(image)./resources/AIDSinfo.gif!,
Tenofovir alafenamide (TAF) ![]() !(image)./resources/AIDSinfo.gif!
!(image)./resources/AIDSinfo.gif!
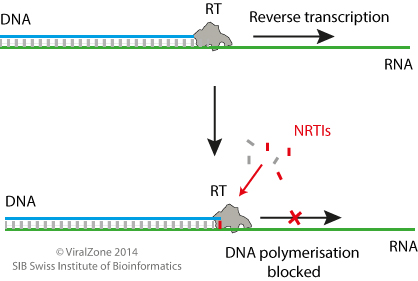
Inhibition mechanism
Once HIV has entered the target host cell reverse transcription, initiated by the viral reverse transcriptase (RT) enzyme, occurs. During reverse transcription the HIV RNA genome is reverse transcribed to DNA which will be transported into the nucleus and integrated into the host genome for subsequent replication. Nucleoside reverse transcriptase inhibitors (NRTIs) are structural nucleoside analogues of DNA nucleotides which prevent reverse transcription of the HIV genome, thereby inhibiting the action of HIV-1 RT and viral replication  . These nucleosides are defective in the sense that they prevent new 3' phosphodiesterase bonds with the next nucleotide in a growing DNA strand
. These nucleosides are defective in the sense that they prevent new 3' phosphodiesterase bonds with the next nucleotide in a growing DNA strand  . These nucleoside analogues are phosphorylated by human cellular kinases to their triphosphate form where they are subsequently incorporated by RT into the growing DNA primer strand of HIV. This results in premature chain termination and the inhibition of successful HIV replication
. These nucleoside analogues are phosphorylated by human cellular kinases to their triphosphate form where they are subsequently incorporated by RT into the growing DNA primer strand of HIV. This results in premature chain termination and the inhibition of successful HIV replication  .
.
Side-effects and metabolism
These drugs have been found highly effective for the treatment of HIV-1 and are generally regarded as safe to use but life-threatening adverse reactions, like mitochondrial toxicity, have been reported  . Mitochondrial toxicity occurs due to the ability of NRTIs, except abacavir and lamivudine, to compete with human mitochondrial DNA polymerase gamma
. Mitochondrial toxicity occurs due to the ability of NRTIs, except abacavir and lamivudine, to compete with human mitochondrial DNA polymerase gamma  . This polymerase is responsible for excision repair of mitochondrial DNA that has been damaged by oxidation. Decrease in mitochondrial DNA excision repair results in a depletion of mitochondrial DNA and the subsequent disruption of oxidative phosphorylation leading to toxin accumulation and mitochondrial toxicity
. This polymerase is responsible for excision repair of mitochondrial DNA that has been damaged by oxidation. Decrease in mitochondrial DNA excision repair results in a depletion of mitochondrial DNA and the subsequent disruption of oxidative phosphorylation leading to toxin accumulation and mitochondrial toxicity  .
.
NRTIs have no CYP 450 interactions  . They are subject to interactions of glucuronidation and absorption and metabolism differs with each drug.
. They are subject to interactions of glucuronidation and absorption and metabolism differs with each drug.
P. G. Hoggard, S. D. Sales, S. Kewn, D. Sunderland, S. H. Khoo, C. A. Hart, D. J. Back
Antivir. Chem. Chemother. November 2000; 11: 353?358
Michael J. Zapor, Kelly L. Cozza, Gary H. Wynn, Glenn W. Wortmann, Scott C. Armstrong
Psychosomatics December 2004; 45: 524?535
Karen S. Anderson
Biochim. Biophys. Acta July 18, 2002; 1587: 296?299
Michael J. Zapor, Kelly L. Cozza, Gary H. Wynn, Glenn W. Wortmann, Scott C. Armstrong
Psychosomatics December 2004; 45: 524?535
G. Moyle
Clin Ther August 2000; 22: 911?936; discussion 898
M. C. McNeely, R. Yarchoan, S. Broder, T. J. Lawley
J. Am. Acad. Dermatol. December 1989; 21: 1213?1217
J. J. Eron, S. L. Benoit, J. Jemsek, R. D. MacArthur, J. Santana, J. B. Quinn, D. R. Kuritzkes, M. A. Fallon, M. Rubin
N. Engl. J. Med. December 21, 1995; 333: 1662?1669
Karen S. Anderson
Biochim. Biophys. Acta July 18, 2002; 1587: 296?299
Peter J. Ruane, David M. Parenti, David M. Margolis, David H. Shepp, Timothy J. Babinchak, Amy S. Van Kempen, Teresa L. Kauf, Susan A. Danehower, Linda Yau, Siegrid M. Hessenthaler, Diane Goodwin, Jaime E. Hernandez, COL30336 Study Team
HIV Clin Trials August 2003; 4: 231?243
Mina John, Elizabeth J. McKinnon, Ian R. James, David A. Nolan, Susan E. Herrmann, Corey B. Moore, Alex J. White, Simon A. Mallal
J. Acquir. Immune Defic. Syndr. May 1, 2003; 33: 29?33
G. J. Moyle, C. Baldwin, B. Langroudi, S. Mandalia, B. G. Gazzard
J. Acquir. Immune Defic. Syndr. May 1, 2003; 33: 22?28
