Integrase inhibitors
Approved:
Dolutegravir (DTG) ![]() !(image)./resources/AIDSinfo.gif!,
Elvitegravir (EVG)
!(image)./resources/AIDSinfo.gif!,
Elvitegravir (EVG) ![]() !(image)./resources/AIDSinfo.gif!,
Raltegravir (RAL)
!(image)./resources/AIDSinfo.gif!,
Raltegravir (RAL) ![]() !(image)./resources/AIDSinfo.gif!
!(image)./resources/AIDSinfo.gif!
Inhibition mechanism
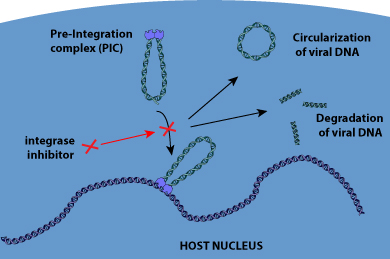
HIV integrase has become an emerging drug target. After reverse transcription, the integrase protein assembles with viral DNA to form a stable complex, termed the pre-integration complex (PIC), and enters the host nucleus. There, integrase mediates the integration of complementary DNA (cDNA) into the host genome in a two-step process. First, two nucleotides are excised from the 3'-ends of viral DNA. Then, the covalent insertion of HIV viral DNA to the host chromosome occurs. Integrase inhibitor Raltegravir interacts with divalent metals within the active site of HIV-1 integrase. This interaction inhibits the insertion of viral DNA into the host chromosome (strand transfer process). Elvitegravir is also a specific inhibitor of the strand-transfer step of HIV integration . Dolutegravir (DTG) is the only second-generation INSTI with FDA approval in 2013
. Dolutegravir (DTG) is the only second-generation INSTI with FDA approval in 2013 .
.
Side-effects and metabolism
The most common side effects of integrase inhibitors are gastrointestinal events, including nausea/vomiting or diarrhea as well as headache, nerveous system and neuropsychiatric effects
Frederick J. Lee, Andrew Carr
Curr Opin HIV AIDS September 2012; 7: 422?428
Emma D. Deeks
Drugs April 2014; 74: 687?697
Jose Luis Blanco Ar?valo, Gary George Whitlock
Expert Opin Pharmacother March 2014; 15: 573?582
