Non-Nucleoside Reverse Transcriptase Inhibitors
Approved, 1st generation:
Efavirenz (EFV) ![]()
 ,
Nevirapine (NVP)
,
Nevirapine (NVP) ![]()
 ,
Delavirdine (DLV)
,
Delavirdine (DLV) ![]()
 Approved, 2nd generation:
Doravirine (DOR)
Approved, 2nd generation:
Doravirine (DOR) ![]()
 ,
Etravirine (ETR)
,
Etravirine (ETR) ![]()
 ,
Rilpivirine (RPV)
,
Rilpivirine (RPV) ![]()
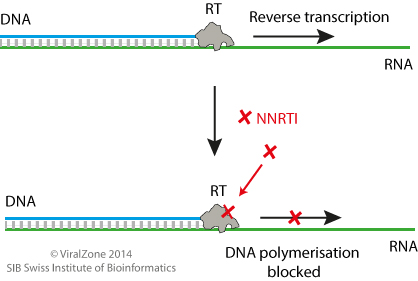
Inhibition mechanism
Once HIV has entered the target host cell reverse transcription, initiated by the viral reverse transcriptase (RT) enzyme, occurs. During reverse transcription the HIV RNA genome is reverse transcribed to DNA which will be transported into the nucleus and integrated into the host genome for subsequent replication. NNRTIs target the reverse transcription step of the HIV life cycle by binding the HIV RT enzyme thereby blocking complementary HIV DNA elongation. NNRTIs directly inhibit the RT enzyme by binding to a pocket residue of the enzyme and inhibit DNA polymerization by inducing allosteric changes to HIV RT at its active site  . This change decreases the RT and nucleoside binding affinity and prevents viral replication
. This change decreases the RT and nucleoside binding affinity and prevents viral replication  .
.
Unlike NRTIs, NNRTIs do not require phosphorylation to be activated and carry out their inhibition mechanism. Furthermore, NNRTIs and NRTIs do not have overlapping sites of resistance mutations and inhibition mechanisms and thus, are usually used together in a cocktail.
Side-effects and metabolism
Toxicities and viral drug resistant mutations do not overlap between NRTIs and NNRTIs, and so are effective together in HAART regimens  . The most common side effects of NNRTIs are fat redistribution
. The most common side effects of NNRTIs are fat redistribution  , elevation of liver enzymes
, elevation of liver enzymes  , and a mild rash
, and a mild rash 
 .
.
Although each NNRTI drug is different in its pharmacokinetics and chemical structure, they share a similar mechanism and are all metabolized by cytochrome P450 enzymes to some extent  . Furthermore, they can inhibit or induce cytochrome P450 enzymes which will affect the metabolism of other drugs.
. Furthermore, they can inhibit or induce cytochrome P450 enzymes which will affect the metabolism of other drugs.
Kalyan Das, Eddy Arnold
Curr Opin Virol April 2013; 3: 111?118
Michael J. Zapor, Kelly L. Cozza, Gary H. Wynn, Glenn W. Wortmann, Scott C. Armstrong
Psychosomatics December 2004; 45: 524?535
P. F. Smith, R. DiCenzo, G. D. Morse
Clin Pharmacokinet 2001; 40: 893?905
J. Balzarini
Curr Top Med Chem 2004; 4: 921?944
J. C. Adkins, S. Noble
Drugs December 1998; 56: 1055?1064; discussion 1065?1066
Douglas T. Dieterich, Patrick A. Robinson, James Love, Jerry O. Stern
Clin. Infect. Dis. March 1, 2004; 38 Suppl 2: S80?89
A. Bardsley-Elliot, C. M. Perry
Paediatr Drugs October 2000; 2: 373?407
L. J. Scott, C. M. Perry
Drugs December 2000; 60: 1411?1444
Kalyan Das, Eddy Arnold
Curr Opin Virol April 2013; 3: 111?118
