Inhibition of host tetherin by virus (kw:KW-1084)
Tetherin is an alpha interferon-inducible cellular factor that impairs the release of many enveloped viruses, including human immunodeficiency
virus type 1 (HIV-1), HIV-2, other retroviruses, Lassa virus-like particles
(VLPs), Marburg and Ebola VLPs.
Tetherin may also act as an innate immune sensor of viral infections that activates NF-kappaB to induce an inflammatory response .
.
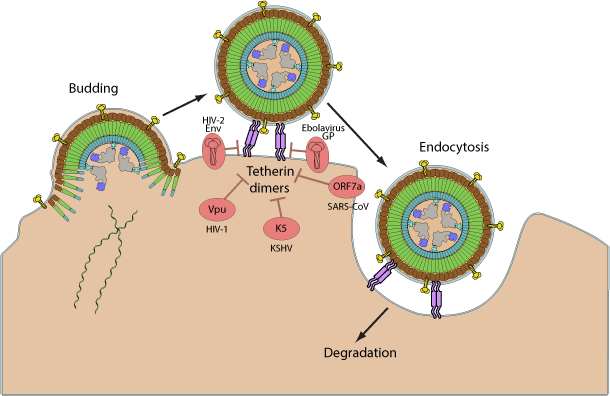
Several viruses manage to circumvent the antiviral activity of tetherin. The Vpu protein form HIV-1 bridges tetherin to BTRC, a substrate recognition subunit of the Skp1/Cullin/F-box protein E3 ubiquitin ligase, inducing its ubiquitination and subsequent proteasomal degradation. HIV-2 instead downregulates cell surface expression of tetherin, restricting it to the trans-Golgi network via direct interaction with the viral glycoprotein.
Carole Bampi, Lara Rasga, Laurent Roux
J. Gen. Virol. June 2013; 94: 1211?1219
Mandana Mansouri, Kasinath Viswanathan, Janet L. Douglas, Jennie Hines, Jean Gustin, Ashlee V. Moses, Klaus Fr?h
J. Virol. October 2009; 83: 9672?9681
Dominik Hotter, Daniel Sauter, Frank Kirchhoff
J. Mol. Biol. December 13, 2013; 425: 4956?4964
Philip H. Jones, Martina Maric, Marisa N. Madison, Wendy Maury, Richard J. Roller, Okeoma Okeoma
Virology March 30, 2013; 438: 37?49
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)61 entries grouped by strain
2 entries
Severe acute respiratory syndrome coronavirus (SARS-CoV) reference strain
2 entries
Severe acute respiratory syndrome coronavirus 2 (2019-nCoV) (SARS-CoV-2) reference strain
1 entry
Human immunodeficiency virus type 1 group M subtype A (isolate MAL) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate ARV2/SF2) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate BRU/LAI) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate HXB2) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate MN) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate RF/HAT3) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group M subtype B (strain 89.6) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group M subtype C (isolate 92BR025) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group M subtype C (isolate ETH2220) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group M subtype D (isolate ELI) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group M subtype F1 (isolate 93BR020) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group M subtype F1 (isolate VI850) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group M subtype H (isolate 90CF056) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group N (isolate YBF30) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 1 group O (isolate ANT70) (HIV-1) reference strain
1 entry
Human immunodeficiency virus type 2 subtype A (isolate BEN) (HIV-2) reference strain
1 entry
Simian immunodeficiency virus (isolate EK505) (SIV-cpz) (Chimpanzee immunodeficiency virus) reference strain
1 entry
Simian immunodeficiency virus (isolate MB66) (SIV-cpz) (Chimpanzee immunodeficiency virus) reference strain
1 entry
Zaire ebolavirus (strain Mayinga-76) (ZEBOV) (Zaire Ebola virus) reference strain
1 entry
Human immunodeficiency virus type 1 (HIV-1)
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate BH10) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate BH8) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate BRVA) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate CDC-451) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate HXB3) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate JH32) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate JRCSF) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate LW123) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate SC) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate SF162) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group M subtype B (isolate YU-2) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group M subtype D (isolate NDK) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group M subtype D (isolate Z2/CDC-Z34) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group N (isolate YBF106) (HIV-1)
1 entry
Human immunodeficiency virus type 1 group O (isolate MVP5180) (HIV-1)
1 entry
Human immunodeficiency virus type 2 subtype A (isolate CAM2) (HIV-2)
1 entry
Human immunodeficiency virus type 2 subtype A (isolate D194) (HIV-2)
1 entry
Human immunodeficiency virus type 2 subtype A (isolate Ghana-1) (HIV-2)
1 entry
Human immunodeficiency virus type 2 subtype A (isolate KR) (HIV-2)
1 entry
Human immunodeficiency virus type 2 subtype A (isolate NIH-Z) (HIV-2)
1 entry
Human immunodeficiency virus type 2 subtype A (isolate ROD) (HIV-2)
1 entry
Human immunodeficiency virus type 2 subtype A (isolate SBLISY) (HIV-2)
1 entry
Human immunodeficiency virus type 2 subtype A (isolate ST) (HIV-2)
1 entry
Human immunodeficiency virus type 2 subtype A (isolate ST/24.1C#2) (HIV-2)
1 entry
Human immunodeficiency virus type 2 subtype B (isolate D205) (HIV-2)
1 entry
Human immunodeficiency virus type 2 subtype B (isolate EHO) (HIV-2)
1 entry
Human immunodeficiency virus type 2 subtype B (isolate UC1) (HIV-2)
1 entry
Reston ebolavirus (strain Philippines-96) (REBOV) (Reston Ebola virus)
1 entry
Reston ebolavirus (strain Reston-89) (REBOV) (Reston Ebola virus)
1 entry
Reston ebolavirus (strain Siena/Philippine-92) (REBOV) (Reston Ebola virus)
1 entry
Sudan ebolavirus (strain Boniface-76) (SEBOV) (Sudan Ebola virus)
1 entry
Sudan ebolavirus (strain Human/Uganda/Gulu/2000) (SEBOV) (Sudan Ebola virus)
1 entry
Sudan ebolavirus (strain Maleo-79) (SEBOV) (Sudan Ebola virus)
1 entry
Tai Forest ebolavirus (strain Cote d'Ivoire-94) (TAFV) (Cote d'Ivoire Ebola virus)
1 entry
Zaire ebolavirus (strain Eckron-76) (ZEBOV) (Zaire Ebola virus)
1 entry
Zaire ebolavirus (strain Gabon-94) (ZEBOV) (Zaire Ebola virus)
1 entry
