Protease inhibitors
Approved, 1st generation:
Amprenavir (APV) ![]() !(image)./resources/AIDSinfo.gif!,
Fosamprenavir (FPV)
!(image)./resources/AIDSinfo.gif!,
Fosamprenavir (FPV) ![]() !(image)./resources/AIDSinfo.gif!,
Indinavir (IDV)
!(image)./resources/AIDSinfo.gif!,
Indinavir (IDV) ![]() !(image)./resources/AIDSinfo.gif!,
Lopinavir (LPV)
!(image)./resources/AIDSinfo.gif!,
Lopinavir (LPV) ![]() ,
Nelfinavir (NFV)
,
Nelfinavir (NFV) ![]() !(image)./resources/AIDSinfo.gif!,
Ritonavir (RTV)
!(image)./resources/AIDSinfo.gif!,
Ritonavir (RTV) ![]() !(image)./resources/AIDSinfo.gif!,
Saquinavir (SQV)
!(image)./resources/AIDSinfo.gif!,
Saquinavir (SQV) ![]() !(image)./resources/AIDSinfo.gif!
Approved, 2nd generation:
Atazanavir (ATV)
!(image)./resources/AIDSinfo.gif!
Approved, 2nd generation:
Atazanavir (ATV) ![]() !(image)./resources/AIDSinfo.gif!,
Darunavir (DRV)
!(image)./resources/AIDSinfo.gif!,
Darunavir (DRV) ![]() !(image)./resources/AIDSinfo.gif!,
Tipranavir (TPV)
!(image)./resources/AIDSinfo.gif!,
Tipranavir (TPV) ![]() !(image)./resources/AIDSinfo.gif!
!(image)./resources/AIDSinfo.gif!
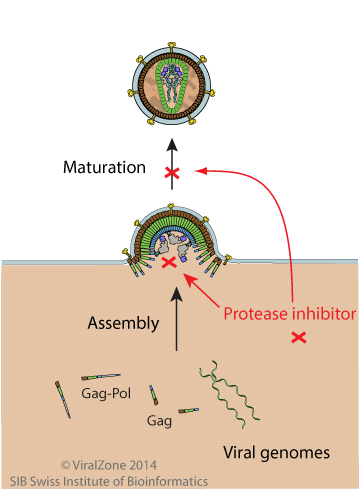
Inhibition mechanism
A very critical step in the HIV-1 replication cycle is the proteolytic cleavage of the viral polypeptide precursors into mature enzymes and structural proteins. This proteolytic activity is absolutely required for the production of mature, infectious virions and is therefore an attractive target for therapeutic intervention. The enzyme performing this task is HIV protease which belongs to the family of aspartyl proteases and possesses an aspartic acid as the active site at position 25. The first protease inhibitors characterized were active site inhibitors based on the structure of natural substrates. They contain a site that cannot be cleaved and thus remain in the catalytic pocket of the enzyme. Unfortunately, the protease enzyme is highly variable and resistance to this treatment can occur. Another strategy is based on the inhibition of protease dimerization. Indeed, to be active the enzyme needs to be folded as a dimer. The targeted region is the anti-parallel beta-barrel formed by N-terminal and C-terminal parts of each monomer. There are currently ten protease inhibitors (PIs) that are US Food and Drug Administration (FDA) approved for clinical use including in highly active antiretroviral therapy (HAART) and all are competitive inhibitors binding at the active site. Tipranavir (TPV) drug inhibits both the enzymatic activity and the dimerization of HIV-1 protease
A. K. Patick, K. E. Potts
Clin. Microbiol. Rev. October 1998; 11: 614?627
Side-effects and metabolism
Hepatotoxicity is the common adverse effect of all US FDA approved HIV protease inhibitors  . HIV protease inhibitors induce diarrhea via different mechanisms including increased calcium-dependent chloride conductance, cellular apoptosis and necrosis as well as decreased proliferation of intestinal epithelial cells
. HIV protease inhibitors induce diarrhea via different mechanisms including increased calcium-dependent chloride conductance, cellular apoptosis and necrosis as well as decreased proliferation of intestinal epithelial cells .
.
R. Bruno, P. Sacchi, L. Maiocchi, S. Patruno, G. Filice
Dig Liver Dis June 2006; 38: 363?373
Hagen Bode, Luzie Lenzner, Oliver Holger Kraemer, Anton Josef Kroesen, Kerstin Bendfeldt, J?rg Dieter Schulzke, Michael Fromm, Gisela Stoltenburg-Didinger, Martin Zeitz, Reiner Ullrich
Antivir. Ther. (Lond.) 2005; 10: 645?655
