Alphaherpesvirinae (taxid:10293)
VIRION
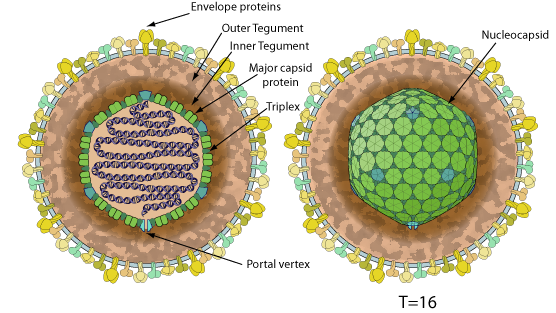
Enveloped, spherical to pleomorphic, 150-200 nm in diameter, T=16 icosahedral symmetry. The capsid consists of 162 capsomers and is surrounded by an amorphous tegument. Glycoproteins complexes are embedded in the lipid envelope.
GENOME
Monopartite, linear, dsDNA genome of 120-180 kb. The genome contains terminal and internal reiterated sequences.
GENE EXPRESSION
Each viral transcript usually encodes a single protein and has a promoter/regulatory sequence, a TATA box, a transcription initiation site, a 5' leader sequence of 30-300 bp (not translated), a 3' nontranslated sequence of 10-30 bp, and a poly A signal. There are many gene overlaps. There are only few spliced genes. Some of the expressed ORFs are antisense to each other. Some ORFs can be accessed from more than one promoter. There are some non-coding genes.
There are three types of genes: immediate-early (?), early(?) and late (?). The ? genes are transcribed immediately after infection and ensure the transcription of ? genes, which encode the proteins necessary for the viral replication. The ? genes mostly encode structural proteins. Standing apart are genes expressed during latency.
The viral proteins VHS and ICP27 are involved in shut-off of host protein translation in order to ensure the selective expression of viral proteins versus cellular proteins.
ENZYMES
- DNA-dependent DNA polymerase
- DNA primase
- Tegument deneddylase (Peptidase C76)
- Assemblin (Peptidase S21)
- Kinase
- Helicase
- Endonuclease
- Ribonucleoside-diphosphate reductase
- Thymidine kinase
- Uracil-DNA glycosylase
REPLICATION
NUCLEAR
Lytic replication:
- Attachment of the viral gB, gC, gD and gH proteins to host receptors mediates endocytosis of the virus into the host cell.
- Fusion with the plasma membrane to release the core and the tegument proteins into the host cytoplasm.
- The capsid is transported to the nuclear pore where viral DNA is released into the nucleus.
- Transcription of immediate early genes which promote transcription of early genes and protect the virus against innate host immunity.
- Transcription of early viral mRNA by host polymerase II, encoding proteins involved in replication of the viral DNA.
- A first round of circular genome amplification occurs by bidirectional replication
- Synthesis of linear concatemer copies of viral DNA by rolling circle.
- Transcription of late mRNAs by host polymerase II, encoding structural proteins.
- Assembly of the virus in nuclear viral factories and budding through the inner lamella of the nuclear membrane which has been modified by the insertion of herpes glycoproteins, throughout the Golgi and final release at the plasma membrane.
Latent replication : replication of circular viral episome in tandem with the host cell DNA using the host cell replication machinery.
Host-virus interaction
Adaptive immune response inhibition
Alpha-herpesviruses have evolved different strategies to inhibit the host adaptive immune response. For example, Herpes simplex protein US12 binds specifically to transporters associated with antigen processing (TAP), blocking peptide-binding to TAP and subsequent loading of peptides onto MHC class I molecules  . VZV ORF66 downregulates MHC I expression by impairing the transport of MHC I molecules from the Golgi compartment to the cell surface
. VZV ORF66 downregulates MHC I expression by impairing the transport of MHC I molecules from the Golgi compartment to the cell surface
Apoptosis modulation
Apoptosis is very often modulated (and usually inhibited) by alpha-herpesviruses. Herpes virus simplex protein US3 activates host protein kinase A/PKA to block apoptosis. Additionally, it also interacts with programmed cell death protein 4 (PDCD4) and retains it in the nucleus thereby inhibiting host apoptosis  .
.
Autophagy modulation
Several alpha-herpesviruses are able to inhibit host cell autophagy process, such as HHV-1 ICP34.5 that interacts with Beclin-1 and stop autophagosomes development  .
.
Cell-cycle modulation
The UL24 protein that is present in all herpesvirus subfamilies (alpha, beta and gamma-herpesviruses) induces a cell cycle arrest at G2/M transition through inactivation of the host cyclinB/cdc2 complex  .
.
Innate immune response inhibition
Herpes viruses inhibit the cascade leading to production of interferon-beta by mainly targeting the host IRF3 protein. Thus, herpes simplex virus or varicella virus possess proteins to prevent IRF3 activation.
Host splicing inhibition
HSV-1 ICP27 is an alternative splicing regulator of host mRNA. This protein is conserved in several herpesviridae genera. It has been shown to act as a splicing silencer at the 3' splice site of the PML intron 7a  .
.
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)0 entry grouped by strain
Bovine herpesvirus 1 taxid:10320
Bovine herpesvirus 1.1 (strain Cooper) taxid:10323
Bovine herpesvirus 1.1 (strain Jura) taxid:31518
| Protein | ModelArchive |
| E3 ubiquitin-protein ligase ICP0 (EC 2.3.2.27) (IER 2.9/ER2.6) (P135 protein) (RING-type E3 ubiquitin transferase... | ma-jd-viral-58609 |
Bovine herpesvirus 1.1 (strain P8-2) taxid:10324
| Protein | ModelArchive |
| Envelope glycoprotein D (gD) (Glycoprotein IV) | ma-jd-viral-07522 |
Bovine herpesvirus type 1.1 taxid:79889
Canid alphaherpesvirus 1 taxid:170325
Cercopithecine alphaherpesvirus 2 taxid:10317
Cercopithecine alphaherpesvirus 9 taxid:35246
Cercopithecine herpesvirus 1 taxid:10325
Cercopithecine herpesvirus 1 (strain E2490) taxid:260965
| Protein | ModelArchive |
| ICP47 protein (Immediate-early protein IE12) (Immediate-early-5) (Infected cell protein 47) (US12 protein)... | ma-jd-viral-62159 |
Cercopithecine herpesvirus 16 taxid:340907
Cercopithecine herpesvirus 9 (strain DHV) taxid:36348
Chimpanzee herpesvirus strain 105640 taxid:332937
Equid alphaherpesvirus 1 taxid:10326
Equid alphaherpesvirus 3 taxid:80341
Equid alphaherpesvirus 4 taxid:10331
Equid alphaherpesvirus 8 taxid:39637
Equid alphaherpesvirus 9 taxid:55744
Equine herpesvirus 1 (strain AB1) taxid:10328
| Protein | ModelArchive |
| Envelope glycoprotein E (gE) | ma-jd-viral-00326 |
| Envelope glycoprotein I | ma-jd-viral-06387 |
| Packaging protein UL32 homolog (Glycoprotein 300) (GP300) | ma-jd-viral-40921 |
Equine herpesvirus 1 (strain Ab4p) taxid:31520
Equine herpesvirus 1 (strain HH1) taxid:310537
| Protein | ModelArchive |
| Uncharacterized gene 1 protein | ma-jd-viral-40542 |
Equine herpesvirus 1 (strain HVS25A) taxid:10327
| Protein | ModelArchive |
| Envelope glycoprotein H (gH) | ma-jd-viral-16908 |
| Thymidine kinase (EC 2.7.1.21) | ma-jd-viral-05904 |
Equine herpesvirus 1 (strain Kentucky A) taxid:10329
Equine herpesvirus 1 (strain Kentucky D) taxid:10330
| Protein | ModelArchive |
| Envelope glycoprotein B (gB) | ma-jd-viral-31223 |
| Envelope glycoprotein C (gC) (Glycoprotein 13) | ma-jd-viral-01823 |
| Envelope protein UL45 homolog | ma-jd-viral-16456 |
Equine herpesvirus 1 (strain V592) taxid:310273
Equine herpesvirus 4 (strain 1942) taxid:10333
Falconid herpesvirus 1 taxid:1510155
Gallid alphaherpesvirus 2 taxid:10390
Gallid alphaherpesvirus 3 taxid:35250
Gallid herpesvirus 2 (strain Chicken/Md5/ATCC VR-987) taxid:10389
Gallid herpesvirus 2 (strain GA) taxid:10388
| Protein | ModelArchive |
| Envelope glycoprotein L (gL) | ma-jd-viral-09079 |
| Virion protein US10 homolog | ma-jd-viral-16121 |
Gallid herpesvirus 2 (strain RB-1b) taxid:33707
| Protein | ModelArchive |
| Envelope glycoprotein B (gB) | ma-jd-viral-31201 |
Hawaiian green turtle herpesvirus taxid:70564
Human herpesvirus 1 taxid:10298
Human herpesvirus 1 (strain 17) taxid:10299
Human herpesvirus 1 (strain MP) taxid:10307
| Protein | ModelArchive |
| Envelope glycoprotein K (Syncytial protein) | ma-jd-viral-49381 |
Human herpesvirus 1 (strain R15) taxid:36345
| Protein | ModelArchive |
| Envelope glycoprotein K (Syncytial protein) | ma-jd-viral-49381 |
Human herpesvirus 2 taxid:10310
Human herpesvirus 2 (strain 333) taxid:10313
Human herpesvirus 2 (strain G) taxid:10314
| Protein | ModelArchive |
| Virion host shutoff protein (Vhs) (EC 3.1.27.-) | ma-jd-viral-22425 |
Human herpesvirus 2 (strain HG52) taxid:10315
Human herpesvirus 3 taxid:10335
Infectious laryngotracheitis virus taxid:10386
Infectious laryngotracheitis virus (strain Thorne V882) taxid:10344
Leporid alphaherpesvirus 4 taxid:481315
Macropodid alphaherpesvirus 1 taxid:137443
Meleagrid herpesvirus 1 taxid:37108
Psittacid herpesvirus 1 (isolate Amazon parrot/-/97-0001/1997) taxid:670426
Pteropodid alphaherpesvirus 1 taxid:1343901
Suid herpesvirus 1 taxid:10345
Suid herpesvirus 1 (strain Indiana-Funkhauser / Becker) taxid:31523
| Protein | ModelArchive |
| Envelope glycoprotein B (gB) | ma-jd-viral-31207 |
| Tripartite terminase subunit 1 | ma-jd-viral-61489 |
Suid herpesvirus 1 (strain Kaplan) taxid:33703
Suid herpesvirus 1 (strain NIA-3) taxid:10349
| Protein | ModelArchive |
| Protein US2 homolog (28 kDa protein) | ma-jd-viral-21068 |
| Serine/threonine-protein kinase UL13 (EC 2.7.11.1) | ma-jd-viral-24774 |
| Tegument protein UL14 | ma-jd-viral-14631 |
Suid herpesvirus 1 (strain Rice) taxid:10350
| Protein | ModelArchive |
| Envelope glycoprotein G (gG) | ma-jd-viral-64437 |
