Inhibition of host PKR by virus (kw:KW-1102)
The protein kinase regulated by RNA, PKR, plays an important function in innate immune response. The N-terminal includes a repeated domain that confers dsRNA-binding activity while the C-terminal confers the kinase activity. The best characterized substrate of PKR is the alpha-subunit of protein synthesis initiator 2 (eIF-2alpha). When phosphorylated by PKR, eIF-2alpha leads to an inhibition of translation. Therefore, PKR is a key factor in the host antiviral response, through inhibition of protein synthesis during viral infection.
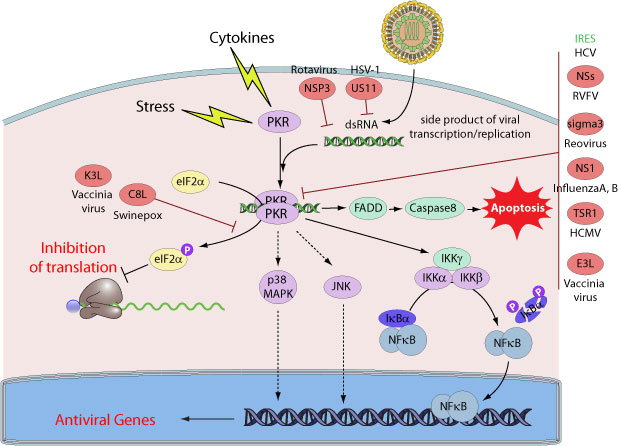
Viruses have evolved specific mechanisms to prevent the establishment of an antiviral state by inhibiting key components of the signaling pathway
 .
For instance, several viral proteins antagonists of PKR have been identified, many of which are RNA-binding proteins. Among them, E3L and K3L from vaccinia and sigma3 protein from reoviruses. E3L was found to inhibit host PKR activity through
direct interaction. Hepatitis C virus NS5A, Herpes simplex 1 US11, and Kaposi's sarcoma vIRF-2 protein also interact with and inhibit PKR. Some viruses including HCMV or MCMV alter PKR subcellular localization while others direct PKR to degradation like Rift valley fever virus NSs protein.
.
For instance, several viral proteins antagonists of PKR have been identified, many of which are RNA-binding proteins. Among them, E3L and K3L from vaccinia and sigma3 protein from reoviruses. E3L was found to inhibit host PKR activity through
direct interaction. Hepatitis C virus NS5A, Herpes simplex 1 US11, and Kaposi's sarcoma vIRF-2 protein also interact with and inhibit PKR. Some viruses including HCMV or MCMV alter PKR subcellular localization while others direct PKR to degradation like Rift valley fever virus NSs protein.
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)178 entries grouped by protein
1 entry
G-protein coupled receptor BILF1 (GPCR BILF1)
1 entry
E1B 55 kDa protein (E1B-55K) (E1B protein, large T-antigen) (E1B-495R)
1 entry
Protein E3 homolog (Protein E3L homolog)
3 entries
mRNA export factor ICP27 homolog (Mta) (ORF57 protein homolog) (Protein EB2) (Protein SM)
2 entries
Protein IRS1
1 entry
Protein K3 homolog
9 entries
Nucleoprotein (Protein N) (Nucleocapsid protein)
135 entries
Non-structural protein 1 (NS1) (NS1A)
2 entries
Non-structural protein NS-S (NSs)
2 entries
Protein K3
4 entries
RNA-binding protein OPG065 (RNA-binding protein E3) (p25)
2 entries
Genome polyprotein
3 entries
Accessory factor US11 (Vmw21)
6 entries
Replicase polyprotein 1ab (ORF1ab polyprotein)
3 entries
Outer capsid protein sigma-3 (Sigma3)
2 entries
Protein HHLF1
1 entry
