Viral initiation of translation
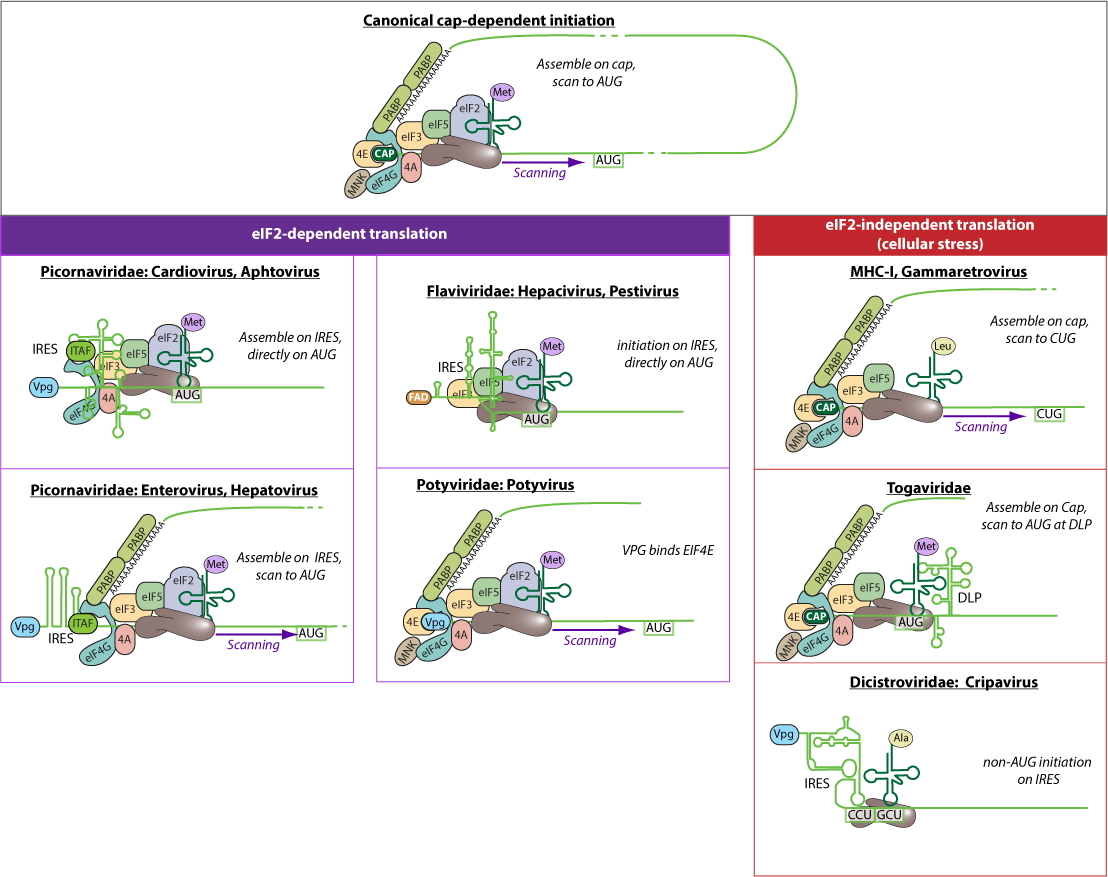
Many viruses have evolved alternative ways to initiate viral mRNA translation, either because the virus shuts off classic host translation, or because the viral RNAs are not classical, i.e. lack a cap or polyadenylation.
Internal Ribosome Entry Site (IRES)  are RNA structures that allow cap-independent initiation of translation and are capable of initiating translation in the middle of a messenger RNA. Cripavirus IRES also allow translation initiation at an alanine or glutamine tRNA.
are RNA structures that allow cap-independent initiation of translation and are capable of initiating translation in the middle of a messenger RNA. Cripavirus IRES also allow translation initiation at an alanine or glutamine tRNA.  Downstream Hairpin Loop (DLP) are RNA structures that allow initiation of cap-dependent translation in the absence of the initiation factor eIF2, where the initiating methionine tRNA is placed on the ribosome by the DLP structure.
Downstream Hairpin Loop (DLP) are RNA structures that allow initiation of cap-dependent translation in the absence of the initiation factor eIF2, where the initiating methionine tRNA is placed on the ribosome by the DLP structure.  CUG initiation involves initiation with a leucine and is more likely to occur under stress with eIF2 inhibition.
CUG initiation involves initiation with a leucine and is more likely to occur under stress with eIF2 inhibition.  .
In VpG initiation, a direct interaction with the 5'-covalently bound protein VPG occurs with EIF4E. This has been described in plants. EIF4E mutations consequently make certain plant varieties resistant to virus infections.
.
In VpG initiation, a direct interaction with the 5'-covalently bound protein VPG occurs with EIF4E. This has been described in plants. EIF4E mutations consequently make certain plant varieties resistant to virus infections.
| Family | Genus | Type Species | GROUP | Initiation codon | Host | References |
| Dicistroviridae | Cripavirus | Cricket paralysis virus CrPV | IRES Group I | Alanine Glutamine |
Insects |  |
| Flaviviridae | Hepacivirus Pestivirus | Hepatitis C virus Bovine diarrhea virus Classical swine fever virus |
IRES Group II | Methionine | Vertebrate |


|
| Nimaviridae | Whispovirus | White spot syndrome virus | IRES | Methionine | Invertebrate |

 |
| Picornaviridae | Cardiovirus Aphthovirus | Encephalomyocarditis virus Foot and mouth disease virus |
IRES Group III | Methionine | Vertebrate |

|
| Picornaviridae | Enterovirus Hepatovirus | Poliovirus Hepatitis A virus |
IRES Group IV | Methionine | Vertebrate |  |
| Polyomaviridae | Polyomavirus | SV40 | IRES | Methionine | Vertebrate |  |
| Retroviridae | Alpharetrovirus | Rous sarcoma virus | IRES | Methionine | Vertebrate | |
| Betaretrovirus | Mouse mammary tumor virus | IRES | Methionine | Vertebrate |  |
|
| Gammaretrovirus | Murine leukemia virus | IRES | Methionine | Vertebrate | ||
| CUG | Leucine | Vertebrate |  |
|||
| Deltaretrovirus | Human T-lymphotropic virus | IRES | Methionine | Vertebrate | ||
| Lentivirus | Human immunodeficiency virus | IRES | Methionine | Vertebrate | ||
| Togaviridae | Alphavirus | Sindbis virus | DLP | Methionine | Vertebrate |  |
| Rubivirus | Rubella virus | DLP | Methionine | Vertebrate |  |
|
| Tombusviridae | Carmovirus | Pelargonium flower break virus | IRES | Methionine | Plant |  |
| Totiviridae | Giardiavirus | Giardia lamblia virus | IRES | Methionine | Protozoa |  |
| Potyviridae | Potyvirus | Lettuce mosaic virus | VpG | Methionine | Plant |  |
Ren? Toribio, Iv?n Ventoso
Proc. Natl. Acad. Sci. U.S.A. May 25, 2010; 107: 9837?9842
A. C. Prats, G. De Billy, P. Wang, J. L. Darlix
J. Mol. Biol. January 20, 1989; 205: 363?372
Shih-Ting Kang, Han-Ching Wang, Yi-Ting Yang, Guang-Hsiung Kou, Chu-Fang Lo
J. Virol. December 2013; 87: 13263-13278
Shih-Ting Kang, Jiann-Horng Leu, Han-Ching Wang, Li-Li Chen, Guang-Hsiung Kou, Chu-Fang Lo
Virology May 10, 2009; 387: 353-363
D. V. Sizova, V. G. Kolupaeva, T. V. Pestova, I. N. Shatsky, C. U. Hellen
J. Virol. June 1998; 72: 4775-782
Susan R. Schwab, Jessica A. Shugart, Tiffany Horng, Subramaniam Malarkannan, Nilabh Shastri
PLoS Biol. November 2004; 2: e366
