Clathrin-mediated endocytosis of virus by host (kw:KW-1165)
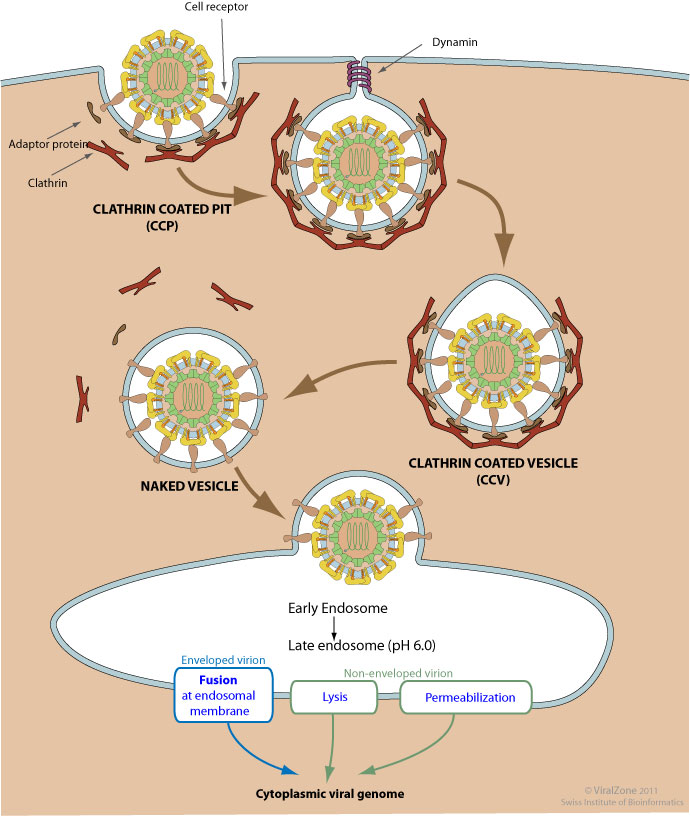
Clathrin-mediated endoycytosis (CME) is triggered by the binding of the virion to host receptors
 .
The virion attachment to the host receptor induces the binding of an adaptor protein to the receptor cytoplasmic tail. Adaptor proteins bind to clathrin, and the local concentration of adaptor proteins on the inside face of the plasma membrane allows clathrin to multimerize to form characteristic invaginations or Clathrin-Coated Pit (CCP). Membrane scission proteins DNM1/Dynamin-1 or DNM2/Dynamin-2 pinch off the CCP from the host membrane thereby releasing the Clathrin-Coated Vesicle (CCV).
.
The virion attachment to the host receptor induces the binding of an adaptor protein to the receptor cytoplasmic tail. Adaptor proteins bind to clathrin, and the local concentration of adaptor proteins on the inside face of the plasma membrane allows clathrin to multimerize to form characteristic invaginations or Clathrin-Coated Pit (CCP). Membrane scission proteins DNM1/Dynamin-1 or DNM2/Dynamin-2 pinch off the CCP from the host membrane thereby releasing the Clathrin-Coated Vesicle (CCV).
The clathrin basket is subsequently released from the vesicle by auxilin and hsc70. The vesicle then delivers its viral content to early endosomes. Endosomal acidic pH and /or receptor binding usually induces structural modifications of the virus surface proteins that leads to genome penetration into the cytoplasm via fusion or permeabilization mechanisms.
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)395 entries grouped by protein
37 entries
Capsid protein VP1 (Coat protein VP1)
3 entries
Penton protein (CP-P) (Penton base protein) (Protein III)
65 entries
Envelope glycoprotein gp160 (Env polyprotein)
12 entries
Glycoprotein
1 entry
Glycoprotein G
3 entries
Envelopment polyprotein (M polyprotein)
1 entry
Glycoprotein 2b (Protein GP2b) (GP(S))
1 entry
Glycoprotein 3 (Protein GP3)
1 entry
Glycoprotein 4 (Protein GP4)
2 entries
Glycoprotein 5 (Protein GP5) (G(L))
123 entries
Hemagglutinin
101 entries
Genome polyprotein
21 entries
Structural polyprotein (p110)
2 entries
Frameshifted structural polyprotein (p130)
2 entries
Envelope glycoprotein p57 (gp84) (gp94)
11 entries
Envelope glycoprotein (GP1,2) (GP)
1 entry
Major capsid protein VP1 (Major structural protein VP1)
8 entries
