HIV and Tuberculosis syndemic
A syndemic is the aggregation of two or more diseases in a population in which there is some level of positive biological interaction that exacerbates the negative health effects of any or all of the diseases.("Wikipedia":https://en.wikipedia.org/wiki/Syndemic)
About one third of deaths among HIV-seropositive patients worldwide are due to co-infection with Mycobacterium tuberculosis (MTB). By infecting the same host, both pathogens replicate much better than alone. This syndemic is induced by a convergent dis-regulation of host immune response by HIV and MTB . HIV infects primarily T-helper lymphocytes while MTB preferentially infects alveolar macrophages; it is likely that the syndemy occurs in trans via circulating factors.
. HIV infects primarily T-helper lymphocytes while MTB preferentially infects alveolar macrophages; it is likely that the syndemy occurs in trans via circulating factors.
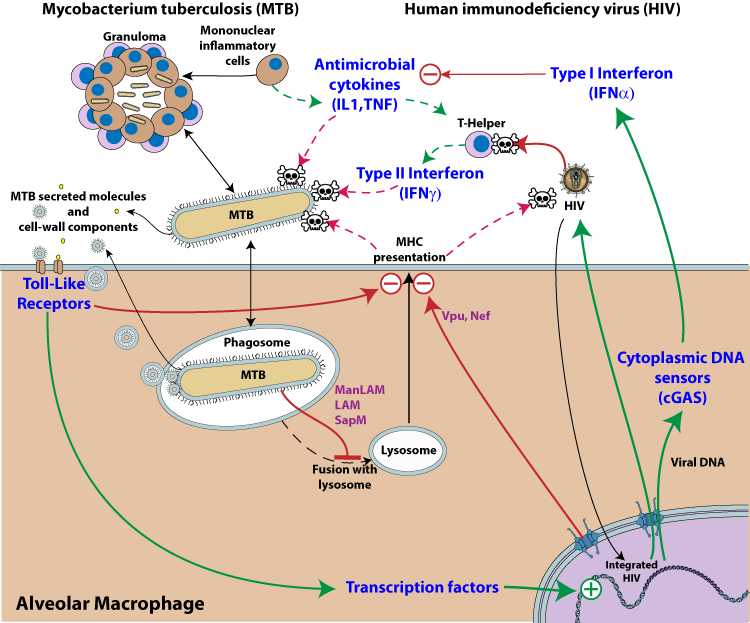
Legend:MTB and HIV Syndemy. Pathway activation is represented by green arrows, whereas inhibition is depicted by red arrows. The dotted lines represent normal host immune inhibition of microbial growth that is down-regulated upon co-infection with MTB and HIV. The two pathogens are represented in the same host cell, but it is likely that the mutualistic effect occurs in trans.
1. HIV activation by MTB
M. tuberculosis infection promotes HIV-1 replication presumably by manipulating cellular transcription factors that regulate HIV-1 transcription  .
.
MTB cell wall components and secreted molecules interact with pattern recognition receptors expressed on phagocytic cells (e.g. Toll-like receptors), triggering various signaling pathways which in turn activate transcription factors such as NF-kappaB  . Those factors bind to the promoter-proximal (enhancer) region of the HIV-1 long terminal repeat (LTR) and induce HIV-1 gene expression
. Those factors bind to the promoter-proximal (enhancer) region of the HIV-1 long terminal repeat (LTR) and induce HIV-1 gene expression  . This results in activation of latent provirus in cells that are reached by MTB secreted molecules.
. This results in activation of latent provirus in cells that are reached by MTB secreted molecules.
2. MTB activation by HIV
Clash of cytokines: Interferon alpha counter-regulates antibacterial cytokines
Interleukin-1 (IL-1), TNF-alpha and interferon-gamma are key cytokines that foster MTB containment  . These essential cytokines are inactivated upon local HIV replication
. These essential cytokines are inactivated upon local HIV replication  . Virus replication triggers a cGAS dependent type I interferon response, which is ineffective against the virus
. Virus replication triggers a cGAS dependent type I interferon response, which is ineffective against the virus  . By up-regulating type I IFN, HIV lowers the host defenses against MTB activity. Type I interferon and interleukin-1/TNF-alpha cytokine pathways represent distinct and specialized categories of inflammatory responses and these key mediators of inflammation counter-regulate each other
. By up-regulating type I IFN, HIV lowers the host defenses against MTB activity. Type I interferon and interleukin-1/TNF-alpha cytokine pathways represent distinct and specialized categories of inflammatory responses and these key mediators of inflammation counter-regulate each other 

 . HIV co-infection also reduces the level of IFN gamma
. HIV co-infection also reduces the level of IFN gamma  , a key element in MTB control by the immune system.
, a key element in MTB control by the immune system.
Modulation of MTB-mediated pulmonary granulomas by HIV co-infection
Granulomas are a means of containing an MTB infection created by the immune system. This inflammatory process is characterized by the presence of macrophages, epithelial cells and multinucleated giant cells that are surrounded by a rim of T-lymphocytes. HIV co-infection modulates the granuloma aspect and cellular composition. De Noronha et al. show that HIV impairs the TNF-alpha production by granuloma cells  . Furthermore, granulomas in HIV/MTB coinfected individuals have fewer lymphocytes
. Furthermore, granulomas in HIV/MTB coinfected individuals have fewer lymphocytes  . Walter et al. found that the presence of HIV infection in patients with tuberculosis was associated primarily with decreased induction of the MTB DosR regulon, which modulates granuloma formation and MTB persistence
. Walter et al. found that the presence of HIV infection in patients with tuberculosis was associated primarily with decreased induction of the MTB DosR regulon, which modulates granuloma formation and MTB persistence  .
.
3. Inhibition of host antigen presentation by HIV and MTB
MTB-antigen processing and presentation are key events in the immune response against MTB. This is stimulated by interferon gamma which is highly secreted by the immune system upon MTB detection. But antigen processing is impaired both by MTB and HIV virus. HIV interferes with antigen presentation through host modulating proteins like vpu, and nef
 .
MTB cell wall components and secreted molecules are able to modulate immune cells and inhibit IFN-gamma secretion
.
MTB cell wall components and secreted molecules are able to modulate immune cells and inhibit IFN-gamma secretion
 . It has been shown that several MTB lipoproteins, including LpqH, LprG and LprA, are key inhibitors of MHC class II antigen presentation, through activation of TLR2
. It has been shown that several MTB lipoproteins, including LpqH, LprG and LprA, are key inhibitors of MHC class II antigen presentation, through activation of TLR2  . HIV also inhibits antigen presentation by redirecting MHC to lysosomes for destruction [Vpu, Nef].
. HIV also inhibits antigen presentation by redirecting MHC to lysosomes for destruction [Vpu, Nef].
Credits: This page has been written in collaboration with Swiss-Prot Prokaryotic protein annotation project and co-funded by the the Swiss Federal Government through the State Secretariat for Education, Research and Innovation SERI and Swiss South African Joint Research Programme (SSJRP).
Lucile Espert, Bruno Beaumelle, Isabelle Vergne
Front Cell Infect Microbiol 2015; 5: 49
Susmita K. Singh, Anna-Maria Andersson, Rada Elleg?rd, Cecilia S. Lindestam Arlehamn, Alessandro Sette, Marie Larsson, Olle Stendahl, Robert Blomgran
Am. J. Pathol. December 2016; 186: 3083?3093
Greta Guarda, Marion Braun, Francesco Staehli, Aubry Tardivel, Chantal Mattmann, Irmgard F?rster, Matthias Farlik, Thomas Decker, Renaud A. Du Pasquier, Pedro Romero, J?rg Tschopp
Immunity February 25, 2011; 34: 213?223
Katrin D. Mayer-Barber, Bo Yan
Cell. Mol. Immunol. June 6, 2016;
Katrin D. Mayer-Barber, Bruno B. Andrade, Sandra D. Oland, Eduardo P. Amaral, Daniel L. Barber, Jacqueline Gonzales, Steven C. Derrick, Ruiru Shi, Nathella Pavan Kumar, Wang Wei, Xing Yuan, Guolong Zhang, Ying Cai, Subash Babu, Marta Catalfamo, Andres M. Salazar, Laura E. Via, Clifton E. Barry, Alan Sher
Nature July 3, 2014; 511: 99?103
Tineke Cantaert, Dominique Baeten, Paul P. Tak, Lisa G. M. van Baarsen
Arthritis Res. Ther. 2010; 12: 219
Jolien Vermeire, Ferdinand Roesch, Daniel Sauter, R?jane Rua, Dominik Hotter, Anouk Van Nuffel, Hanne Vanderstraeten, Evelien Naessens, Veronica Iannucci, Alessia Landi, Wojciech Witkowski, Ann Baeyens, Frank Kirchhoff, Bruno Verhasselt
Cell Rep October 4, 2016; 17: 413?424
J. Nigou, C. Zelle-Rieser, M. Gilleron, M. Thurnher, G. Puzo
J. Immunol. June 15, 2001; 166: 7477?7485
Alm?rio L. L. de Noronha, Andr? B?fica, Lucas Nogueira, Aldina Barral, Manoel Barral-Netto
Pathol. Res. Pract. 2008; 204: 155?161
Collin R. Diedrich, Jennifer O?Hern, Maximiliano G. Gutierrez, Nafiesa Allie, Patricia Papier, Graeme Meintjes, Anna K. Coussens, Helen Wainwright, Robert J. Wilkinson
J. Infect. Dis. July 26, 2016;
Nicholas D. Walter, Bouke C. de Jong, Benjamin J. Garcia, Gregory M. Dolganov, William Worodria, Patrick Byanyima, Emmanuel Musisi, Laurence Huang, Edward D. Chan, Tran T. Van, Martin Antonio, Abigail Ayorinde, Midori Kato-Maeda, Payam Nahid, Ann M. Leung, Andrew Yen, Tasha E. Fingerlin, Katerina Kechris, Michael Strong, Martin I. Voskuil, J. Lucian Davis, Gary K. Schoolnik
J. Infect. Dis. October 15, 2016; 214: 1205?1211
Smriti Mehra, Taylor W. Foreman, Peter J. Didier, Muhammad H. Ahsan, Teresa A. Hudock, Ryan Kissee, Nadia A. Golden, Uma S. Gautam, Ann-Marie Johnson, Xavier Alvarez, Kasi E. Russell-Lodrigue, Lara A. Doyle, Chad J. Roy, Tianhua Niu, James L. Blanchard, Shabaana A. Khader, Andrew A. Lackner, David R. Sherman, Deepak Kaushal
Am. J. Respir. Crit. Care Med. May 15, 2015; 191: 1185?1196
Susmita K. Singh, Anna-Maria Andersson, Rada Elleg?rd, Cecilia S. Lindestam Arlehamn, Alessandro Sette, Marie Larsson, Olle Stendahl, Robert Blomgran
Am. J. Pathol. December 2016; 186: 3083?3093
P. Fairman, J. B. Angel
Clin. Exp. Immunol. October 2012; 170: 101?113
Candice K. Kwan, Joel D. Ernst
Clin. Microbiol. Rev. April 2011; 24: 351?376
Dennis Wong, Horacio Bach, Jim Sun, Zakaria Hmama, Yossef Av-Gay
Proc Natl Acad Sci U S A November 29, 2011; 108: 19371?19376
Johanneke Kleinnijenhuis, Marije Oosting, Leo A. B. Joosten, Mihai G. Netea, Reinout Van Crevel
Clin. Dev. Immunol. 2011; 2011: 405310
Dennis Wong, Joseph D. Chao, Yossef Av-Gay
Trends Microbiol. February 2013; 21: 100?109
Clifford V. Harding, W. Henry Boom
Nat. Rev. Microbiol. April 2010; 8: 296?307
Z. Toossi, L. Xia, M. Wu, A. Salvekar
Clin. Exp. Immunol. August 1999; 117: 324?330
James V. Falvo, Shahin Ranjbar, Luke D. Jasenosky, Anne E. Goldfeld
Am. J. Respir. Cell Mol. Biol. December 2011; 45: 1116?1124
Jillian L. M. Waruk, Zipporah Machuki, Christine Mesa, Jennifer A. Juno, Omu Anzala, Meenu Sharma, T. Blake Ball, Julius Oyugi, Sandra Kiazyk
Tuberculosis (Edinb) September 2015; 95: 555?561
John D. MacMicking
Cold Spring Harb Perspect Med July 31, 2014; 4
