Percavirus (taxid:548688)
VIRION
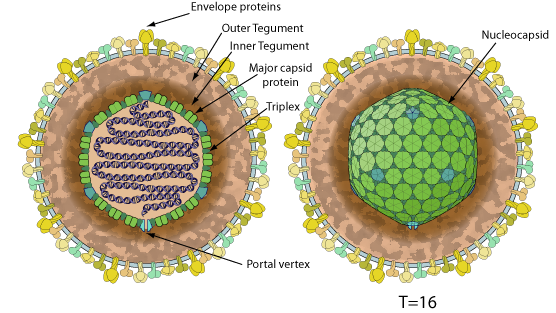
Enveloped, spherical to pleomorphic, 150-200 nm in diameter, T=16 icosahedral symmetry. Capsid consists of 162 capsomers and is surrounded by an amorphous tegument. Glycoproteins complexes are embeded in the lipid envelope.
GENOME
Monopartite, linear, dsDNA genome of about 180 kb. The genome contains terminal and internal reiterated sequences.
GENE EXPRESSION
Each viral transcript usually encodes a single protein and has a promoter/regulatory sequence, a TATA box, a transcription initiation site, a 5' leader sequence of 30-300 bp (not translated), a 3' nontranslated sequence of 10-30 bp, and a poly A signal. There are many gene overlaps. There are only few spliced genes. Some of the expressed ORFs are antisense to each other. Some ORFs can be accessed from more than one promoter. There are some non-coding genes.
ENZYMES
- DNA-dependent DNA polymerase
- DNA primase
- Tegument deneddylase (Peptidase C76)
- Assemblin (Peptidase S21)
- Kinase
- Helicase
- Ribonucleoside-diphosphate reductase
- Thymidine kinase
- Uracil-DNA glycosylase
REPLICATION
NUCLEAR
Lytic replication:
- Attachment of the viral glycoproteins to host receptors mediates endocytosis of the virus into the host cell.
- Fusion with the plasma membrane to release the core and the tegument proteins into the host cytoplasm.
- The capsid is transported to the nuclear pore where the viral DNA is released into the nucleus.
- Transcription of immediate early genes which promote transcription of early genes and protect the virus against innate host immunity.
- Transcription of early viral mRNA by host polymerase II, encoding proteins involved in replication of the viral DNA.
- A first round of circular genome amplification occurs by bidirectional replication
- Synthesis of linear concatemer copies of viral DNA by rolling circle.
- Transcription of late mRNAs by host polymerase II, encoding structural proteins.
- Assembly of the virus in nuclear viral factories and budding through the inner lamella of the nuclear membrane which has been modified by the insertion of herpes glycoproteins, throughout the Golgi and final release at the plasma membrane.
Latent replication : replication of circular viral episome in tandem with the host cell DNA using the host cell replication machinery.
Host-virus interaction
Adaptive immune response inhibition
The viral interleukin-10 homolog down-regulates the expression of the TAP1 gene (transporter associated with antigen processing), thereby affecting the transport of peptides into the endoplasmic reticulum and subsequent peptide loading by MHC class I molecules.
Apoptosis modulation
The EHV-2 v-FLIP protein (ORF E8) inhibits TNFRSF1A, TNFRSF6, TNFRSF10 and TNFRSF12 induced apoptosis.
Cell-cycle modulation
The UL24 protein induces a cell cycle arrest at G2/M transition through inactivation of the host cyclinB/cdc2 complex  .
.
Splicing inhibition
Modulates the viral and host mRNA expression by exporting unspliced mRNA, thereby inducing alternative splicing.
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)75 entries grouped by strain
74 entries
Equine herpesvirus 2 (strain 86/87) (EHV-2) reference strain
1 entry
Equine herpesvirus 2 (strain T400/3) (EHV-2)
Equid gammaherpesvirus 2 taxid:12657
Equid gammaherpesvirus 5 taxid:10371
Equine herpesvirus 2 (strain 86/87) taxid:82831
Equine herpesvirus 2 (strain T400/3) taxid:82832
| Protein | ModelArchive |
| Viral interleukin-10 homolog (vIL-10) | ma-jd-viral-13428 |
