Inhibition of host IKBKE by virus (kw:KW-1224)
Upon activation of viral RNA sensors, such as RIG-I-like receptors, IKK-epsilon associates with DDX3X and phosphorylates interferon regulatory factors (IRFs), IRF3 and IRF7, as well as DDX3X. This activity allows subsequent homodimerization and nuclear translocation of the IRF3 leading to transcriptional activation of pro-inflammatory and antiviral genes including IFNB. In order to establish such an antiviral state, IKBKE forms several different complexes whose composition depends on the type of cell and cellular stimuli.
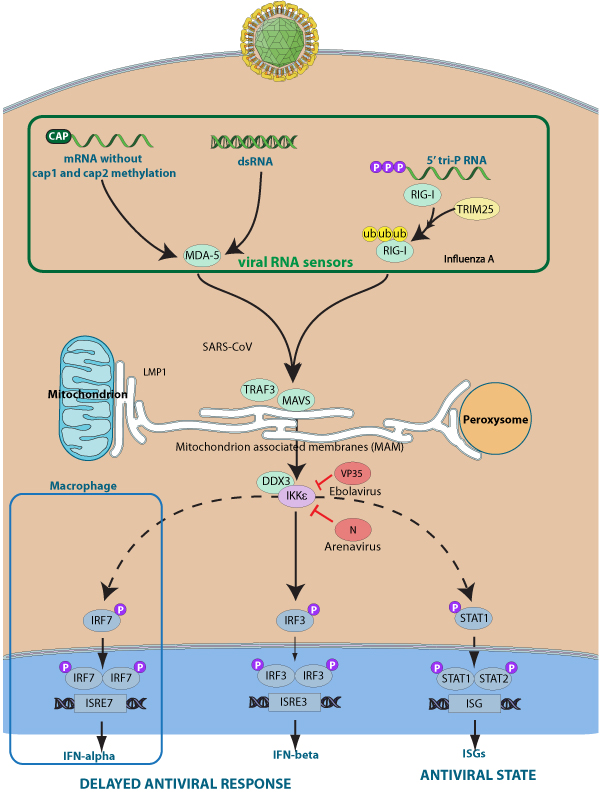
Several viruses including arenaviruses or ebola virus interact directly with and inhibit IKBKE/IKK-epsilon to prevent IRFs activation. Engagement of arenavirus nucleocapsid protein seems to sequester IKKepsilon in an inactive complex while VP35 from ebola virus interferes with the interaction between Ikkepsilon and IRF3/IRF7 substrates.
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)32 entries grouped by strain
1 entry
Alethinophid 1 reptarenavirus (isolate AlRrV1/Boa/USA/BC/2009) (Golden Gate virus) reference strain
1 entry
Bat coronavirus HKU4 (BtCoV) (BtCoV/HKU4/2004) reference strain
1 entry
Bat coronavirus HKU5 (BtCoV) (BtCoV/HKU5/2004) reference strain
1 entry
Cupixi mammarenavirus (isolate Rat/Brasil/BeAn 119303/1970) (CPXV) reference strain
1 entry
Lassa virus (strain Mouse/Sierra Leone/Josiah/1976) (LASV) reference strain
1 entry
Latino mammarenavirus (isolate Rat/Bolivia/MARU 1924/1965) (LATV) reference strain
1 entry
Lymphocytic choriomeningitis virus (strain Armstrong) (LCMV) reference strain
1 entry
Middle East respiratory syndrome-related coronavirus (isolate United Kingdom/H123990006/2012) (MERS-CoV) (Betacoronavirus England 1) reference strain
1 entry
Oliveros mammarenavirus (isolate Mouse/Argentina/RIID 3229/1990) (OLVV) reference strain
1 entry
Parana mammarenavirus (isolate Rat/Paraguay/12056/1965) (PARV) (Paran mammarenavirus) reference strain
1 entry
Pirital mammarenavirus (isolate Rat/Venezuela/VAV-488/1995) (PIRV) reference strain
1 entry
Tacaribe virus (strain Franze-Fernandez) (TCRV) reference strain
1 entry
Whitewater Arroyo mammarenavirus (isolate Rat/United States/AV 9310135/1995) (WWAV) reference strain
1 entry
Zaire ebolavirus (strain Mayinga-76) (ZEBOV) (Zaire Ebola virus) reference strain
1 entry
Allpahuayo mammarenavirus (isolate Rat/Peru/CLHP-2472/1997) (ALLV)
1 entry
Bear Canyon mammarenavirus (isolate Mouse/United States/AV A0070039/2000) (BCNV)
1 entry
Chapare mammarenavirus (isolate Human/Bolivia/810419/2003)
1 entry
Guanarito mammarenavirus (isolate Human/Venezuela/NH-95551/1990) (GTOV)
1 entry
Ippy mammarenavirus (isolate Rat/Central African Republic/Dak An B 188 d/1970) (IPPYV)
1 entry
Junin mammarenavirus (JUNV) (Junn mammarenavirus)
1 entry
Lassa virus (strain GA391) (LASV)
1 entry
Lymphocytic choriomeningitis virus (strain WE) (LCMV)
1 entry
Machupo virus (MACV)
1 entry
Mobala mammarenavirus (isolate Rat/Central African Republic/Acar 3080/1983) (MOBV)
1 entry
Mopeia virus (MOPV)
1 entry
Pichinde mammarenavirus (PICV) (Pichind mammarenavirus)
1 entry
Reston ebolavirus (strain Philippines-96) (REBOV) (Reston Ebola virus)
1 entry
Reston ebolavirus (strain Reston-89) (REBOV) (Reston Ebola virus)
1 entry
Sabia mammarenavirus (isolate Human/Brasil/SPH114202/1990) (SABV) (Sabi mammarenavirus)
1 entry
Sudan ebolavirus (strain Human/Uganda/Gulu/2000) (SEBOV) (Sudan Ebola virus)
1 entry
Tamiami mammarenavirus (isolate Rat/United States/W 10777/1964) (TAMV)
1 entry
