Modulation of host ubiquitin pathway by virus (kw:KW-1130)
Numerous cellular processes are regulated by the reversible conjugation of ubiquitin (UB) or ubiquitin-like (UBL) proteins to substrates. Typically, a ubiquitin-activating enzyme (E1) binds ubiquitin in an adenosine triphosphate dependent manner. Ubiquitin is then transferred to a ubiquitin-conjugating enzyme (E2). A ubiquitin ligase (E3) helps transfer ubiquitin to the target substrate. In turn, polyubiquitination targets proteins to a cellular organelle known as the 26S proteasome where substrates are degraded into small peptides. Proteasomal degradation is critical component of many cellular processes including cell cycle regulation,induction of the inflammatory response and antigen presentation.
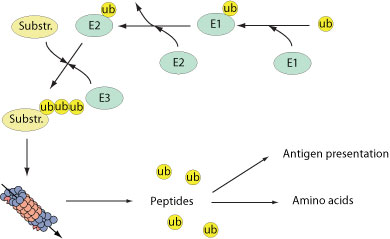
Viruses have evolved different strategies to modulate host cell ubiquitin machinery. The reasons of this manipulation are multiple including modulation of the host cell cycle, viral replication, inhibition of the host immune system or viral budding. The modification of the ub system includes the modulation of cellular E3 ligases, the synthesis of viral E3 ligases, viral deubiquitinases, or viral ubiquitin-like proteins that will perturb normal host Ub pathways.
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)155 entries grouped by protein
7 entries
Early E1A protein (Early E1A 27 kDa protein)
1 entry
Early 4 ORF6 protein (E4-ORF6) (Early 4 34 kDa protein) (E4-34k)
1 entry
E3 ubiquitin-protein ligase IAP-3 (EC 2.3.2.27) (IAP-3) (RING-type E3 ubiquitin transferase IAP-3)
7 entries
E3 ubiquitin-protein ligase ICP0 (EC 2.3.2.27) (IER 2.9/ER2.6) (P135 protein) (RING-type E3 ubiquitin transferase ICP0)
1 entry
E3 ubiquitin-protein ligase IE2 (EC 2.3.2.27) (Immediate-early protein IE2) (RING-type E3 ubiquitin transferase IE2)
2 entries
E3 ubiquitin-protein ligase IE61 (EC 2.3.2.27) (Immediate-early protein 61) (IE61)
7 entries
Kelch repeat and BTB domain-containing protein 1
4 entries
Kelch repeat and BTB domain-containing protein 2
2 entries
RNA-directed RNA polymerase L (Protein L) (Large structural protein) (Replicase) (Transcriptase)
6 entries
E3 ubiquitin-protein ligase LAP (EC 2.3.2.27) (Leukemia associated protein) (LAP) (RING-type E3 ubiquitin transferase LAP)
3 entries
Latent membrane protein 2 (Terminal protein)
1 entry
Large T antigen (LT) (LT-AG) (EC 5.6.2.4) (DNA 3'-5' helicase large T antigen)
23 entries
Large tegument protein deneddylase (EC 3.4.19.12) (EC 3.4.22.-)
1 entry
Ankyrin repeat domain-containing protein M-T5 (M-T5 protein) (M5)
8 entries
Host range factor p28 (EC 2.3.2.27) (E3 ubiquitin-protein ligase p28)
3 entries
Ankyrin repeat domain-containing protein OPG023 (Host range protein 1)
1 entry
Genome polyprotein
8 entries
Non-structural replication polyprotein
26 entries
Replicase polyprotein 1a (pp1a) (ORF1a polyprotein)
27 entries
Replicase polyprotein 1ab (pp1ab) (ORF1ab polyprotein)
7 entries
Replicase polyprotein 1ab (ORF1ab polyprotein)
1 entry
RING finger containing E3 ubiquitin-protein ligase WSV222 (EC 2.3.2.27) (RING-type E3 ubiquitin transferase WSV222)
1 entry
RING finger containing E3 ubiquitin-protein ligase WSV403 (EC 2.3.2.27) (RING-type E3 ubiquitin transferase WSV403)
2 entries
Ubiquitin-like protein
5 entries
