Modulation of host ubiquitin pathway by viral E3 ligase (kw:KW-1128)
Numerous cellular processes are regulated by the reversible conjugation of ubiquitin (UB) or ubiquitin-like (UBL) proteins to substrates. The E3 ubiquitin ligase operates in conjunction with an E1 ubiquitin-activating enzyme and an E2 ubiquitin-conjugating enzyme and transfers ubiquitin to the target substrate.
Two types of E3 ubiquitin ligases are present in cells based on the structure of their catalytic domain named RING and HECT. The E3s polyubiquitinate target proteins to a cellular organelle known as the 26S proteasome where substrates are degraded into small peptides. Proteasomal degradation is critical component of many cellular processes including cell cycle regulation,induction of the inflammatory response and antigen presentation.
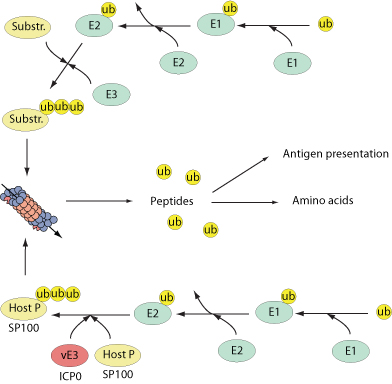
Several viruses encode viral E3 ligases in order to direct specific substrates for proteasomal degradation. For example, herpes simplex virus encodes ICPO which acts as a nuclear E3 ubiquitin ligase that polyubiquitinates the nuclear bodies-associated PML and Sp100 proteins.
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)33 entries grouped by protein
1 entry
Early 4 ORF6 protein (E4-ORF6) (Early 4 34 kDa protein) (E4-34k)
1 entry
E3 ubiquitin-protein ligase IAP-3 (EC 2.3.2.27) (IAP-3) (RING-type E3 ubiquitin transferase IAP-3)
7 entries
E3 ubiquitin-protein ligase ICP0 (EC 2.3.2.27) (IER 2.9/ER2.6) (P135 protein) (RING-type E3 ubiquitin transferase ICP0)
1 entry
E3 ubiquitin-protein ligase IE2 (EC 2.3.2.27) (Immediate-early protein IE2) (RING-type E3 ubiquitin transferase IE2)
2 entries
E3 ubiquitin-protein ligase IE61 (EC 2.3.2.27) (Immediate-early protein 61) (IE61)
6 entries
E3 ubiquitin-protein ligase LAP (EC 2.3.2.27) (Leukemia associated protein) (LAP) (RING-type E3 ubiquitin transferase LAP)
8 entries
Host range factor p28 (EC 2.3.2.27) (E3 ubiquitin-protein ligase p28)
1 entry
RING finger containing E3 ubiquitin-protein ligase WSV222 (EC 2.3.2.27) (RING-type E3 ubiquitin transferase WSV222)
1 entry
RING finger containing E3 ubiquitin-protein ligase WSV403 (EC 2.3.2.27) (RING-type E3 ubiquitin transferase WSV403)
5 entries
