Modulation of host ubiquitin pathway by viral deubiquitinase (kw:KW-1127)
Numerous cellular processes are regulated by the reversible conjugation of ubiquitin (UB) or ubiquitin-like (UBL) proteins to substrates. The human genome encodes several DUBs or deubiquitinating enzymes which oppose the function of E3 ligases. Several keys functions have been attributed to deubiquitinating enzymes:
- Deubiquitination can rescue proteins from degradation
- Deubiquitination can remove a non degradative signal used by cells
- Action together with E3 ligases maintain the normal intracellular pool of ubiquitin.
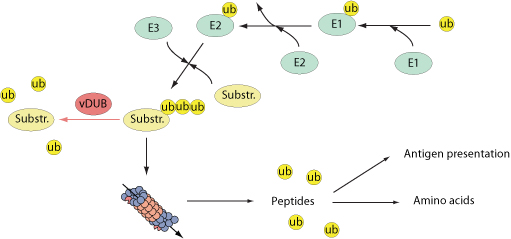
Several viruses encode viral deubiquitinases. One of the functions of these viral deubiquitinases is to prevent host innate response activation. For example, EBV-encoded BPLF1 interacts with and deubiquitinates TRAF6 to inhibit NF-kB signaling during viral infection. KSHV ORF64, a protein with deubiquitinase (DUB) activity, suppresses RIG-I-mediated IFN signaling by reducing the ubiquitination of RIG-I, essential for its activation. PLP2 protein from murine hepatitis virus, strongly inhibits cellular type I interferon production.
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)94 entries grouped by protein
2 entries
RNA-directed RNA polymerase L (Protein L) (Large structural protein) (Replicase) (Transcriptase)
23 entries
Large tegument protein deneddylase (EC 3.4.19.12) (EC 3.4.22.-)
1 entry
Genome polyprotein
8 entries
Non-structural replication polyprotein
26 entries
Replicase polyprotein 1a (pp1a) (ORF1a polyprotein)
27 entries
Replicase polyprotein 1ab (pp1ab) (ORF1ab polyprotein)
7 entries
