Inhibition of host MAVS by virus (kw:KW-1097)
The MAVS protein is required for innate immune defense against viruses. It acts downstream of RIG-I and MDA5, which detect intracellular dsRNA produced during viral replication, to coordinate pathways leading to induction of antiviral cytokines. Alternatively, MAVS can cause apoptosis independent of its function in initiating interferon production. MAVS harbors a C-terminal transmembrane domain that targets it to the mitochondrial outer membrane.
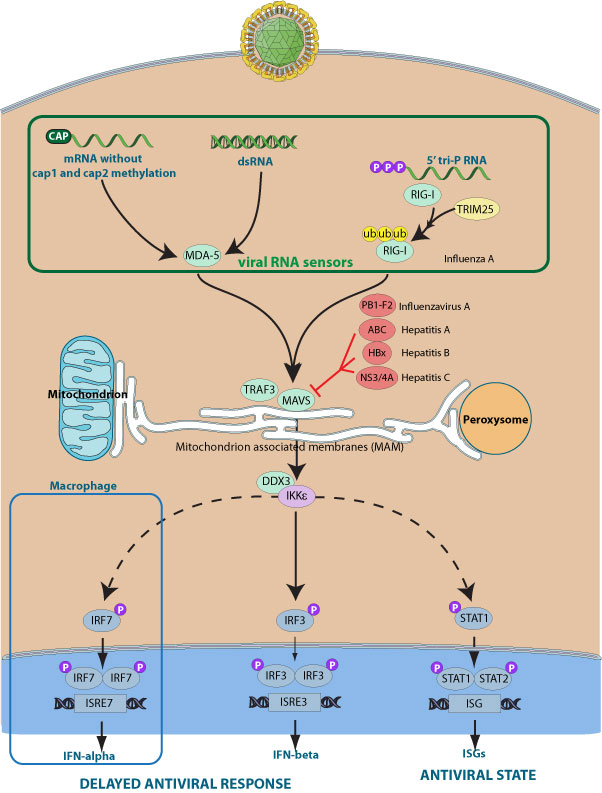
Some viruses selectively inhibit MAVS function. For example, Hepatitis C virus encodes a specific serine protease NS3/4A which cleaves the MAVS transmembrane domain, removes it from the mitochondria thereby preventing interferon production. In a similar way, Hepatitis A encodes for the ABC protein, which localizes to the mitochondria and inhibits MAVS signaling via proteolytic cleavage.
Zsuzsanna T Varga, Alesha Grant, Balaji Manicassamy, Peter Palese
J. Virol. August 2012; 86: 8359?8366
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)210 entries grouped by protein
6 entries
Envelopment polyprotein (Glycoprotein precursor) (M polyprotein)
1 entry
Protein M2-2
7 entries
Nucleoprotein (Protein N) (Nucleocapsid protein)
7 entries
Non-structural protein 1 (Non-structural protein 1C)
1 entry
Non-structural protein NS-S (NSs)
2 entries
ORF9b protein (Accessory protein 9b) (ORF-9b) (Protein 9b)
56 entries
Protein PB1-F2
54 entries
Polymerase basic protein 2 (RNA-directed RNA polymerase subunit P3)
76 entries
