Chymotrypsin fold
Chymotrypsin-fold proteins form icosahedral T=4 capsids built from two beta-barrels resembling the chymotrypsin protease fold. They function as proteases that catalyze their own release from the structural polyprotein.
Origin
The chymotrypsin fold is present in all cellular organisms as well as in many viruses. In the Togaviridae, the capsid and surface proteins show similarity to the NS3 protease and the envelope protein of the Flaviviridae, respectively. This suggests that an ancestral RNA segment from a Flaviviridae-like virus may have combined with an Alsuviricetes polymerase polyprotein, giving rise to a T=4 capsid that evolved from the chymotrypsin protease fold.
Topology
The chymotrypsin fold consists of two beta-barrels, at the interface of which lies the catalytic site His/(Asp, Glu)/(Ser, Cys) .
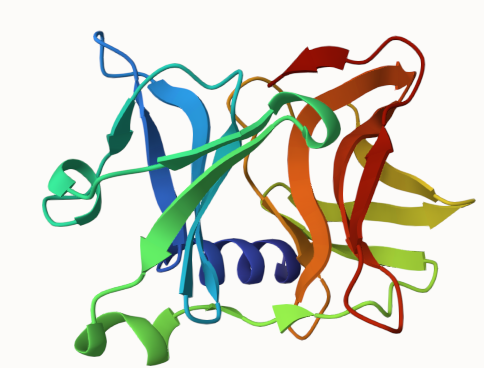
Sindbis capsid 1KXD
