RetroCA-fold
RetroCA-fold proteins form icosahedral capsids characteristic of reverse-transcribing viruses. These proteins are structurally flexible and can assemble into hexamers or pentamers, thereby generating capsids of diverse shapes and sizes .
Origin
The N-terminal domain is unique to reverse-transcribing viruses, while the C-terminal domain is homologous to the SCAN domain (PF02023), a protein?interaction module found in vertebrate transcription factors. The evolutionary origin of this domain remains unclear .
Topology
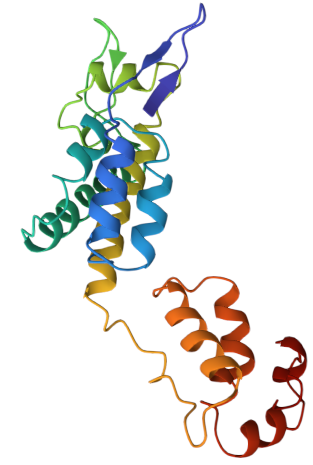
The fold comprises two domains:
- An N-terminal domain of seven alpha-helices, which assembles into pentamers or hexamers.
- A C-terminal domain of four alpha-helices, which mediates interactions between CA multimers to form the complete capsid.
HIV-1 CA 3DIK
