Degradation of host lipopolysaccharides during virus entry (kw:KW-1237)
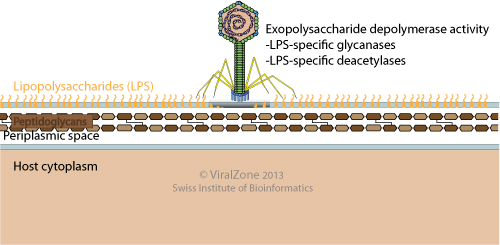
Some bacterial viruses carry an enzymatic activity to degrade the lipopolysaccharides (LPS) of Gram-negative bacteria. This activity is often associated with the tailspike proteins. LPS degradation may be utilized by the bacteriophage to clear a path through the bacterial LPS to gain access to the entry receptor on the cell surface. Interestingly, LPS degrading viruses can also use LPS as attachment receptor. In that case an alternative theory is that LPS degradation may act to release progeny phage particles from cellular debris at the end of a lytic cycle.
| Virus | Family | Host bacteria | Surface component type | Phage degrading enzyme | Ref. |
| Phage HK620 | Podoviridae | Escherichia coli H | LPS | Tail spike endo-N-acetylglucosaminidase |  |
| Phage P27 | Unclassified | Salmonella typhimurium | LPS | Endorhamnosidase |  |
| Phage 9NA | Unclassified | Salmonella typhimurium | LPS | Endorhamnosidase |  |
| Phage KB-1 | Unclassified | Salmonella typhimurium | LPS | Endorhamnosidase |  |
| Phage Det7 | Myoviridae | Salmonella typhimurium | LPS | Endorhamnosidase |  |
| Phage P22 | Podoviridae | Salmonella typhimurium | LPS | Endorhamnosidase |  |
| Phage Sf6 | Podoviridae | Shigella flexneri | LPS | Endorhamnosidase |  |
| Phage & epsilon 15 | Podoviridae | LPS | Endorhamnosidase |  |
Crystal structure of Escherichia coli phage HK620 tailspike: podoviral tailspike endoglycosidase modules are evolutionarily related
Stefanie Barbirz, Jurgen J Muller, Charlotte Uetrecht, Alvin J Clark, Udo Heinemann, Robert Seckler
Mol. Microbiol. July 2008; 69: 303-316
Stefanie Barbirz, Jurgen J Muller, Charlotte Uetrecht, Alvin J Clark, Udo Heinemann, Robert Seckler
Mol. Microbiol. July 2008; 69: 303-316
Salmonella bacteriophage glycanases: endorhamnosidase activity of bacteriophages P27, 9NA, and KB1
R Wollin, U Eriksson, A A Lindberg
J. Virol. June 1981; 38: 1025-1033
R Wollin, U Eriksson, A A Lindberg
J. Virol. June 1981; 38: 1025-1033
Structure of the receptor-binding protein of bacteriophage det7: a podoviral tail spike in a myovirus
Monika Walter, Christian Fiedler, Renate Grassl, Manfred Biebl, Reinhard Rachel, X Lois Hermo-Parrado, Antonio L Llamas-Saiz, Robert Seckler, Stefan Miller, Mark J van Raaij
J. Virol. March 2008; 82: 2265-2273
Monika Walter, Christian Fiedler, Renate Grassl, Manfred Biebl, Reinhard Rachel, X Lois Hermo-Parrado, Antonio L Llamas-Saiz, Robert Seckler, Stefan Miller, Mark J van Raaij
J. Virol. March 2008; 82: 2265-2273
The Shigella flexneri bacteriophage Sf6 tailspike protein (TSP)/endorhamnosidase is related to the bacteriophage P22 TSP and has a motif common to exo- and endoglycanases, and C-5 epimerases
J E Chua, P A Manning, R Morona
Microbiology (Reading, Engl.) July 1999; 145 ( Pt 7): 1649-1659
J E Chua, P A Manning, R Morona
Microbiology (Reading, Engl.) July 1999; 145 ( Pt 7): 1649-1659
In vitro interaction between phage and receptor lipopolysaccharide: a novel glycosidase associated with Salmonella phage epsilon15
K Takeda, H Uetake
Virology March 1973; 52: 148-159
K Takeda, H Uetake
Virology March 1973; 52: 148-159
Matching UniProtKB/Swiss-Prot entries
(all links/actions below point to uniprot.org website)3 entries grouped by protein
3 entries
